Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemicals
2.3. Saccharina Latissima Samples
2.4. Salmon Sampling
2.4.1. Experiment 1: Salmon storage with seaweed and dripped liquid
2.4.2. Experiment 2: Salmon storage with seaweed and limited amount of dripped liquid
2.5. Extraction
2.5.1. Extraction of Polar Metabolites from Salmon Muscle by TCA
2.5.2. Extraction of Polar Metabolites from Saccharina Latissima by Water
2.5.3. Extraction of Non-Polar Metabolites from Salmon Muscle by Acetone
2.6. NMR Sample Preparation
2.6.1. Salmon Muscle and Algal Polar Extracts
2.6.2. Algal Juice
2.6.3. Salmon Muscle Acetone Extract
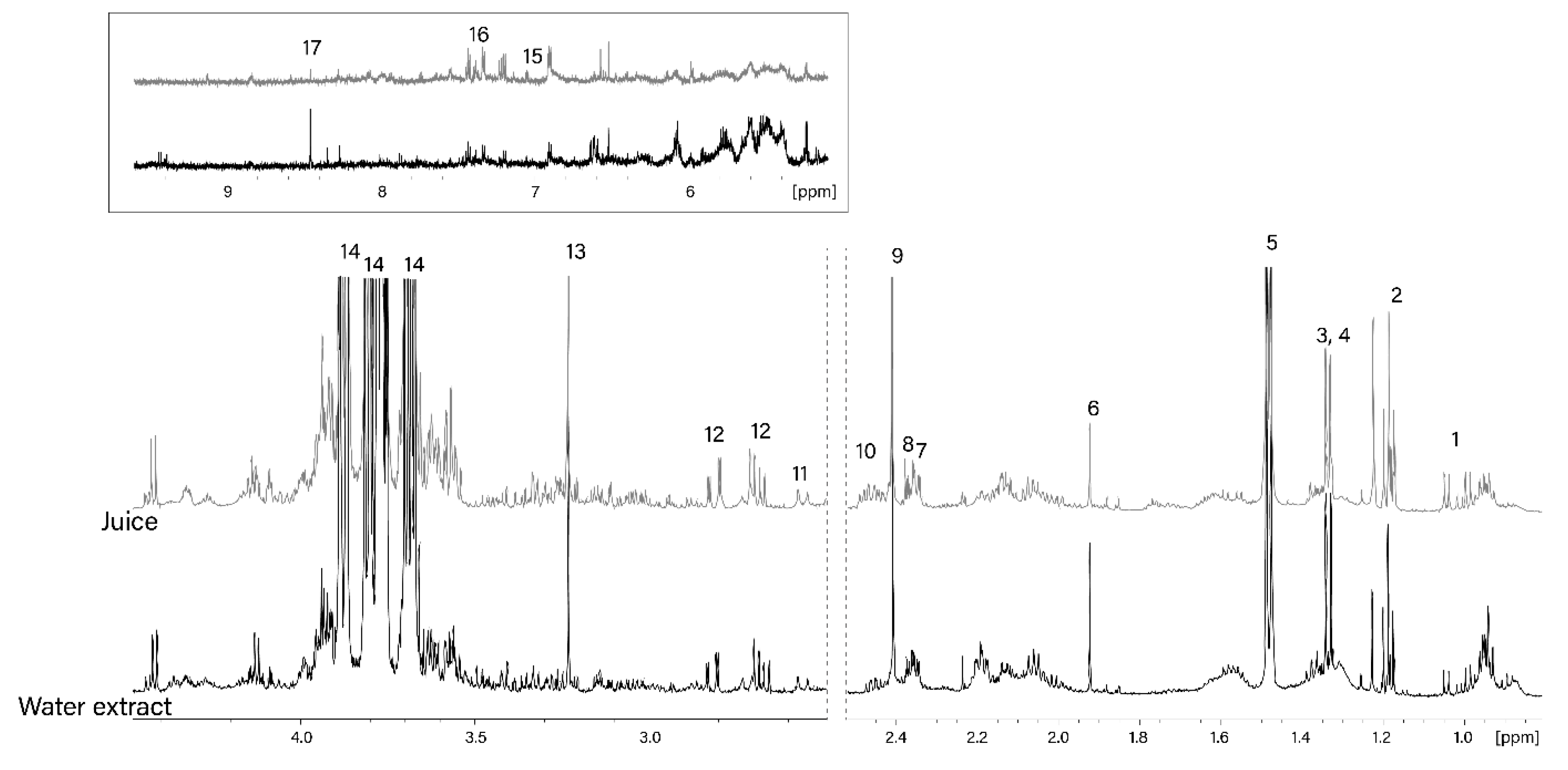
2.7. NMR Data Acquisition and Processing
2.8. Statistical Data Analysis
3. Results and Discussion
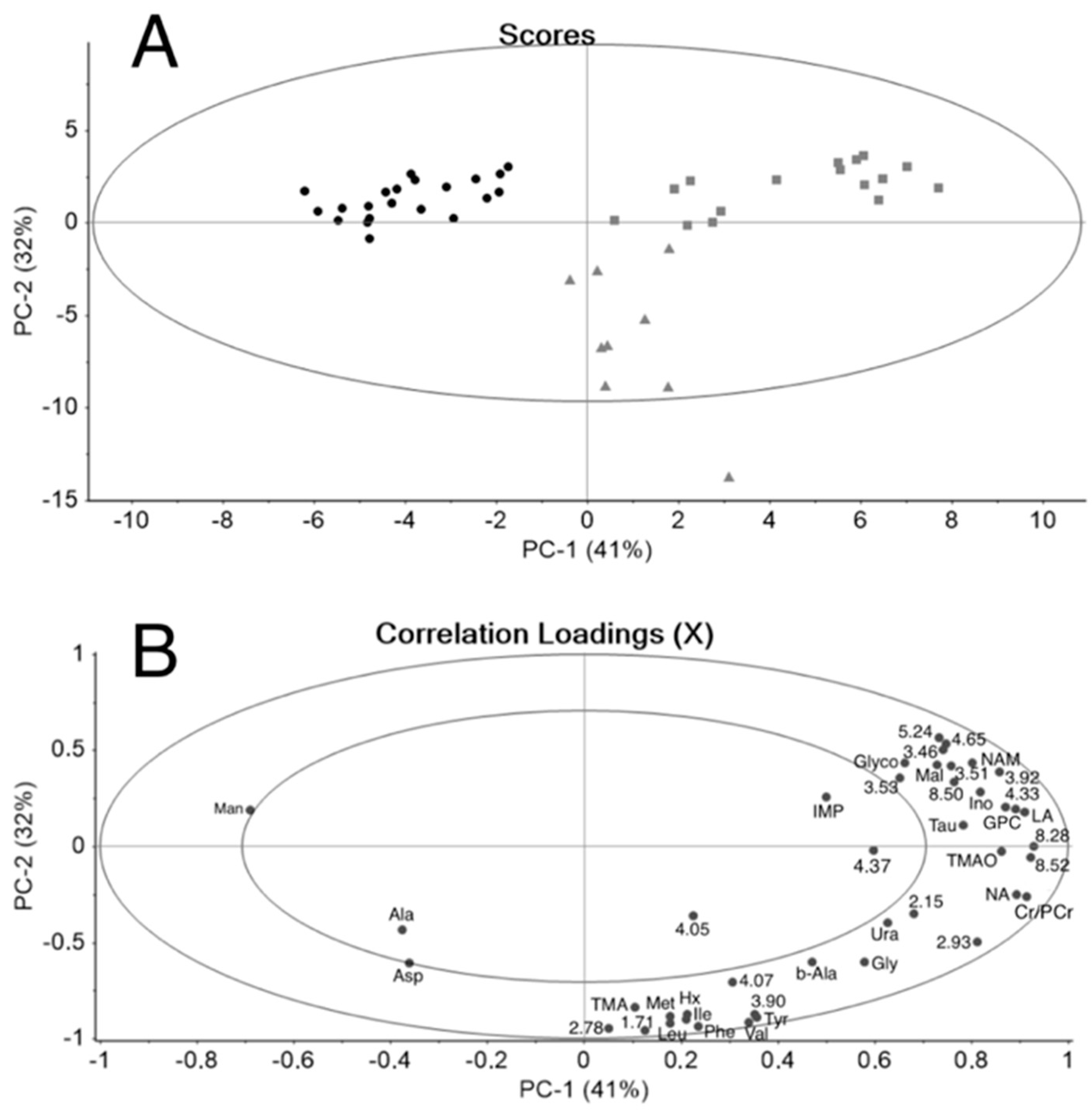
3.1. Principal Component Analysis
3.2. 3-Part System
3.2.1. Polar Metabolites from Sugar Kelp and Its Juice
3.2.2. Changes in the Salmon Metabolome after the Addition of Sugar Kelp
3.3. Shelf Life of Atlantic Salmon
3.3.1. Formation of Trimethylamine (TMA)
3.3.2. Formation of Biogenic Amines
3.4. Flavor Enrichment
3.5. Carotenoids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| Ala | Alanine |
| AMP | Adenosine monophosphate |
| Asp | Asparate/aspartic acid |
| ATP | Adenosine triphosphate |
| b-Ala | β-Alanine |
| Cr/PCr | Creatine/phosphocreatine |
| D2O | Deuterium oxide |
| Glu | Glutamate/glutamic acid |
| Gly | Glycine |
| Glyco | Glycogen |
| GPC | Glycerophosphocholine |
| Hx | Hypoxanthine |
| Hz | Hertz |
| Ile | Isoleucine |
| IMP | Inosine-5′-monophosphate |
| Ino | Inosine |
| LA | Lactate/lactic acid |
| Leu | Leucine |
| Mal | Maltose |
| Man | Mannitol |
| Met | Methionine |
| NA | Nicotinate/nicotinic acid |
| NAM | Niacinamide |
| NMR | Nuclear Magnetic Resonance |
| NS | Number of scans |
| PCA | Principal component analysis |
| Phe | Phenylalanine |
| ppm | Parts per million |
| RG | Receiver gain |
| RS | Reference samples |
| S. latissima | Saccharina latissima |
| STS | Seaweed-treated samples |
| SW | Spectral width |
| Tau | Taurine |
| TCA | Trichloroacetic acid |
| Thr | Threonine |
| TMA | Trimethylamine |
| TMAO | Trimethylamine oxide |
| TMS | Tetramethylsilane |
| TSP | Trimethylsilylpropionic acid |
| Tyr | Tyrosine |
| Ura | Uracil |
| Val | Valine |
References
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsøyen, E. Alginate as a Source of Dietary Fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, K. Seaweeds: A sustainable food source. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 347–364. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Stévant, P.; Rebours, C.; Chapman, A. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Sterner, M.; Edlund, U. Multicomponent fractionation of Saccharina latissima brown algae using chelating salt solutions. J. Appl. Phycol. 2016, 28, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbø, R.; Lock, E.-J.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Gupta, S.; Rajauria, G.; Abu-Ghannam, N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 482–489. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N. Growth inhibition of common food spoilage and pathogenic microorganisms in the presence of brown seaweed extracts. Food Bioprocess Technol. 2012, 5, 1907–1916. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibanez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Arulkumar, A.; Paramasivam, S.; Miranda, J.M. Combined effect of icing medium and red alga Gracilaria verrucosa on shelf life extension of Indian Mackerel (Rastrelliger kanagurta). Food Bioprocess Technol. 2018, 11, 1911–1922. [Google Scholar] [CrossRef]
- Shumilina, E.; Ciampa, A.; Capozzi, F.; Rustad, T.; Dikiy, A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 C. Food Chem. 2015, 184, 12–22. [Google Scholar] [CrossRef]
- Shumilina, E.; Slizyte, R.; Mozuraityte, R.; Dikiy, A. Monitoring of Quality Changes in Salmon and Salmon Rest Raw Materials by NMR. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Germany, 2017; pp. 1–16. [Google Scholar] [CrossRef]
- Shumilina, E.; Slizyte, R.; Mozuraityte, R.; Dykyy, A.; Stein, T.A.; Dikiy, A. Quality changes of salmon by-products during storage: Assessment and quantification by NMR. Food Chem. 2016, 211, 803–811. [Google Scholar] [CrossRef]
- Castejon, D.; Villa, P.; Calvo, M.M.; Santa-Maria, G.; Herraiz, M.; Herrera, A. 1H-HRMAS NMR study of smoked Atlantic salmon (Salmo salar). Magn. Reson. Chem. 2010, 48, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.-M. Considerations of sample preparation for metabolomics investigation. In the Handbook of Metabolomics; Fan, T.M., Lane, A., Higashi, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 7–27. [Google Scholar] [CrossRef]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2007, 36, D402–D408. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO: Rome, Italy, 1995. [Google Scholar]
- Saito, T.; Arai, K.; Matuyoshi, M. A new method for estimating the freshness of fish. Bull. Jap. Soc. Sci. Fish. 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Van Waarde, A. Biochemistry of non-protein nitrogenous compounds in fish including the use of amino acids for anaerobic energy production. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 91, 207–228. [Google Scholar] [CrossRef]
- Hebard, C.E.; Flick, G.J.; Martin, R.E. Occurrence and significance of trimethylamine oxide and its derivatives in fish and shellfish. Chem. Biochem. Mar. Food Prod. 1982, 149–304. [Google Scholar]
- Ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in ‘t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Paulsen, P.; Grossgut, R.; Bauer, F.; Rauscher-Gabernig, E. Estimates of maximum tolerable levels of tyramine content in foods in Austria. J. Food Nutr. Res. 2012, 51, 52–59. [Google Scholar]
- Rauscher-Gabernig, E.; Gabernig, R.; Brueller, W.; Grossgut, R.; Bauer, F.; Paulsen, P. Dietary exposure assessment of putrescine and cadaverine and derivation of tolerable levels in selected foods consumed in Austria. Eur. Food Res. Technol. 2012, 235, 209–220. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. What is umami? Food Rev. Int. 1998, 14, 123–138. [Google Scholar] [CrossRef]
- Müller, V. Bacterial Fermentation. eLS 2001. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; Food and Agricultural Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Kuchiba-Manabe, M.; Matoba, T.; Hasegawa, K. Sensory Changes in Umami Taste of Inosine 5′-Monophosphate Solution after Heating. J. Food Sci. 1991, 56, 1429–1432. [Google Scholar] [CrossRef]
- Hughes, R.B.; Jones, N.R. Measurement of hypoxanthine concentration in canned herring as an index of the freshness of the raw material, with a comment on flavour relations. J. Sci. Food Agric. 1966, 17, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613. [Google Scholar] [CrossRef]

| Chemical Shift of Bucket (ppm) | Compound Assignment | p Value | f Value |
|---|---|---|---|
| Cluster of RS | |||
| 0.96 | Leucine | 0.010 | 7.188 |
| 1.01 | Isoleucine | 0.009 | 7.488 |
| 1.04 | Valine | 0.000 | 19.307 |
| 1.71 | − | 0.009 | 7.457 |
| 2.14 | Methionine | 0.028 | 5.138 |
| 2.15 | − | 0.000 | 23.691 |
| 2.56 | β-Alanine | 0.000 | 38.144 |
| 2.78 | − | 0.031 | 4.937 |
| 2.90 | TMA | 0.007 | 8.018 |
| 2.93 | − | 0.000 | 416.485 |
| 3.04 | Phospho/creatine | 0.000 | 1046.988 |
| 3.23 | Glycerophosphocholine | 0.000 | 40.301 |
| 3.27 | TMAO | 0.000 | 115.006 |
| 3.42 | Taurine | 0.000 | 23.640 |
| 3.46 | − | 0.011 | 6.957 |
| 3.51 | − | 0.005 | 8.856 |
| 3.53 | − | 0.018 | 6.081 |
| 3.56 | Glycine | 0.000 | 35.586 |
| 3.90 | − | 0.000 | 27.242 |
| 3.92 | − | 0.000 | 25.875 |
| 4.05 | − | 0.002 | 11.365 |
| 4.07 | − | 0.000 | 30.336 |
| 4.12 | Lactic acid | 0.000 | 93.297 |
| 4.28 | Inosine | 0.000 | 33.667 |
| 4.33 | − | 0.000 | 30.980 |
| 4.37 | − | 0.001 | 13.929 |
| 4.65 | − | 0.009 | 7.439 |
| 5.24 | − | 0.018 | 6.013 |
| 5.38 | Glycogen | 0.020 | 5.792 |
| 5.40 | Maltose | 0.005 | 8.540 |
| 7.19 | Tyrosine | 0.000 | 17.817 |
| 7.43 | Phenylalanine | 0.001 | 11.838 |
| 7.55 | Uracil | 0.000 | 17.794 |
| 7.60 | Niacinamide | 0.000 | 29.455 |
| 8.20 | Hypoxanthine | 0.000 | 16.858 |
| 8.28 | − | 0.000 | 103.470 |
| 8.50 | − | 0.000 | 19.896 |
| 8.52 | − | 0.000 | 136.123 |
| 8.58 | IMP | 0.017 | 6.122 |
| 8.94 | Nicotinic acid | 0.000 | 734.601 |
| Cluster of STS | |||
| 1.48 | Alanine | 0.013 | 6.731 |
| 2.80 | Aspartic acid | 0.038 | 4.578 |
| 3.80 | Mannitol | 0.000 | 73.052 |
| Compound | Concentration (mg/100 g) |
|---|---|
| Acetate | 1.62 ± 0.00 |
| Alanine | 52.78 ± 0.01 |
| Aspartate | 5.70 ± 0.00 |
| Citrate | 4.31 ± 0.00 |
| Ethanol | 2.57 ± 0.00 |
| Formate | 0.30 ± 0.00 |
| Glutamate | 13.57 ± 0.01 |
| Glutamine | 4.66 ± 0.00 |
| Glycerophosphocholine (GPC) | 6.37 ± 0.00 |
| Isoleucine | 0.60 ± 0.00 |
| Mannitol | 644.92 ± 0.02 |
| Phenylalanine | 0.79 ± 0.00 |
| Pyruvate | 0.03 ± 0.00 |
| Succinate | 7.01 ± 0.00 |
| Tyrosine | 0.55 ± 0.00 |
| Valine | 1.55 ± 0.00 |
| Concentration (mg/100 g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after Slaughter | ||||||||||||||||
| Experiment 1 | ||||||||||||||||
| Compound | Par. | 5 | 7 | 9 | 11 | 14 | 17 | 19 | 21 | |||||||
| RS | RS | STS | RS | STS | RS | STS | RS | STS | RS | STS | RS | STS | RS | STS | ||
| Alanine | 1 | 35.82 ± 0.01 | 39.76 ± 0.01 | 74.68 ± 0.01 | 40.45 ± 0.01 | 48.85 ± 0.00 | 45.89 ± 0.00 | 49.65 ± 0.00 | 35.12 ± 0.01 | 75.45 ± 0.01 | 39.96 ± 0.01 | 62.81 ± 0.01 | 32.73 ± 0.01 | 31.62 ± 0.00 | 58.30 ± 0.03 | 42.69 ± 0.01 |
| 2 | 28.70 ± 0.01 | 32.22 ± 0.01 | 48.95 ± 0.01 | 32.82 ± 0.01 | 43.70 ± 0.01 | 39.71 ± 0.00 | 50.02 ± 0.01 | 31.67 ± 0.00 | 58.00 ± 0.01 | 39.31 ± 0.01 | 57.76 ± 0.00 | 46.83 ± 0.03 | 50.77 ± 0.01 | 55.80 ± 0.01 | 55.74 ± 0.01 | |
| 3 | 38.78 ± 0.01 | 42.63 ± 0.02 | 50.95 ± 0.01 | 48.96 ± 0.01 | 81.42 ± 0.02 | 43.20 ± 0.02 | 58.45 ± 0.00 | 43.65 ± 0.00 | 73.09 ± 0.00 | 25.99 ± 0.01 | 61.51 ± 0.01 | 39.84 ± 0.00 | 87.92 ± 0.01 | 62.04 ± 0.00 | 39.03 ± 0.00 | |
| Anserine | 1 | 671.55 ± 0.01 | 728.22 ± 0.01 | 304.35 ± 0.02 | 714.22 ± 0.00 | 319.10 ± 0.01 | 700.59 ± 0.03 | 223.65 ± 0.01 | 572.00 ± 0.01 | 292.02 ± 0.00 | 631.43 ± 0.00 | 194.52 ± 0.01 | 580.65 ± 0.01 | 216.93 ± 0.01 | 554.70 ± 0.02 | 244.89 ± 0.01 |
| 2 | 652.74 ± 0.00 | 565.03 ± 0.02 | 252.94 ± 0.01 | 575.58 ± 0.00 | 305.21 ± 0.00 | 617.29 ± 0.00 | 218.53 ± 0.01 | 539.32 ± 0.01 | 263.32 ± 0.01 | 570.32 ± 0.01 | 220.17 ± 0.01 | 593.39 ± 0.01 | 127.99 ± 0.01 | 520.58 ± 0.00 | 200.97 ± 0.00 | |
| 3 | 774.76 ± 0.03 | 751.42 ± 0.01 | 341.72 ± 0.01 | 757.25 ± 0.01 | 347.63 ± 0.01 | 687.16 ± 0.01 | 205.95 ± 0.02 | 647.99 ± 0.01 | 198.59 ± 0.01 | 637.31 ± 0.01 | 194.94 ± 0.03 | 574.51 ± 0.01 | 208.04 ± 0.00 | 600.39 ± 0.00 | 195.33 ± 0.01 | |
| Aspartate | 1 | 0.00 | 0.00 | 4.17 ± 0.00 | 0.00 | 3.30 ± 0.00 | 0.00 | 1.67 ± 0.00 | 0.00 | 3.79 ± 0.00 | 0.00 | 3.19 ± 0.00 | 1.61 ± 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 0.00 | 0.00 | 3.37 ± 0.00 | 0.00 | 2.78 ± 0.00 | 0.00 | 3.34 ± 0.000 | 0.00 | 5.47 ± 0.000 | 0.00 | 3.54 ± 0.00 | 0.00 | 0.96 ± 0.00 | 3.20 ± 0.00 | 0.00 | |
| 3 | 0.00 | 0.00 | 2.60 ± 0.01 | 0.00 | 2.14 ± 0.00 | 0.00 | 3.64 ± 0.00 | 0.00 | 6.06 ± 0.00 | 0.00 | 0.89 ± 0.00 | 0.00 | 1.91 ± 0.00 | 0.00 | 0.64 ± 0.01 | |
| β-Alanine | 1 | 3.35 ± 0.00 | 5.00 ± 0.00 | 4.48 ± 0.00 | 12.45 ± 0.01 | 2.87 ± 0.00 | 19.48 ± 0.00 | 2.30 ± 0.00 | 18.97 ± 0.01 | 3.68 ± 0.00 | 14.36 ± 0.00 | 8.99 ± 0.00 | 14.27 ± 0.00 | 5.62 ± 0.01 | 22.51 ± 0.01 | 4.17 ± 0.00 |
| 2 | 2.58 ± 0.00 | 4.77 ± 0.00 | 1.08 ± 0.00 | 6.61 ± 0.00 | 3.37 ± 0.00 | 9.04 ± 0.00 | 2.37 ± 0.00 | 13.17 ± 0.00 | 4.09 ± 0.00 | 11.73 ± 0.00 | 5.16 ± 0.00 | 8.53 ± 0.00 | 4.44 ± 0.00 | 12.97 ± 0.00 | 4.58 ± 0.00 | |
| 3 | 2.57 ± 0.00 | 4.95 ± 0.00 | 1.52 ± 0.00 | 9.32 ± 0.00 | 4.31 ± 0.00 | 11.85 ± 0.00 | 1.99 ± 0.00 | 16.77 ± 0.00 | 3.65 ± 0.00 | 6.88 ± 0.00 | 5.89 ± 0.00 | 9.99 ± 0.00 | 4.55 ± 0.00 | 14.46 ± 0.00 | 4.85 ± 0.00 | |
| Betaine | 1 | 8.58 ± 0.00 | 10.00 ± 0.00 | 2.41 ± 0.00 | 10.46 ± 0.00 | 4.46 ± 0.00 | 12.52 ± 0.00 | 3.68 ± 0.00 | 14.42 ± 0.00 | 4.10 ± 0.00 | 22.06 ± 0.00 | 3.86 ± 0.00 | 29.49 ± 0.01 | 7.70 ± 0.00 | 28.82 ± 0.00 | 8.92 ± 0.00 |
| 2 | 7.21 ± 0.00 | 6.81 ± 0.00 | 2.31 ± 0.00 | 7.18 ± 0.00 | 3.62 ± 0.00 | 7.14 ± 0.00 | 2.41 ± 0.00 | 12.82 ± 0.00 | 4.01 ± 0.00 | 27.85 ± 0.00 | 3.40 ± 0.00 | 36.83 ± 0.01 | 2.59 ± 0.00 | 47.02 ± 0.00 | 9.89 ± 0.00 | |
| 3 | 8.02 ± 0.01 | 10.06 ± 0.00 | 3.42 ± 0.00 | 10.26 ± 0.00 | 5.29 ± 0.00 | 9.84 ± 0.01 | 2.53 ± 0.00 | 10.16 ± 0.00 | 2.75 ± 0.00 | 38.93 ± 0.00 | 6.80 ± 0.00 | 32.35 ± 0.00 | 4.73 ± 0.00 | 40.16 ± 0.01 | 9.16 ± 0.00 | |
| Creatine (mmol/100 g) | 1 | 3.77 ± 0.15 | 3.99 ± 0.02 | 1.48 ± 0.01 | 3.96 ± 0.06 | 1.57 ± 0.02 | 3.37 ± 0.03 | 1.07 ± 0.02 | 3.09 ± 0.05 | 1.41 ± 0.05 | 3.58 ± 0.03 | 1.15 ± 0.01 | 3.38 ± 0.02 | 1.32 ± 0.00 | 3.02 ± 0.09 | 1.31 ± 0.05 |
| 2 | 3.63 ± 0.08 | 3.51 ± 0.01 | 1.36 ± 0.02 | 3.41 ± 0.02 | 1.59 ± 0.02 | 3.43 ± 0.02 | 1.09 ± 0.02 | 3.32 ± 0.03 | 1.46 ± 0.02 | 3.43 ± 0.04 | 1.19 ± 0.06 | 3.70 ± 0.00 | 0.74 ± 0.01 | 3.01 ± 0.00 | 1.22 ± 0.02 | |
| 3 | 3.47 ± 0.00 | 3.76 ± 0.03 | 1.52 ± 0.00 | 3.97 ± 0.07 | 1.62 ± 0.04 | 3.55 ± 0.01 | 0.96 ± 0.03 | 3.43 ± 0.04 | 0.95 ± 0.01 | 3.18 ± 0.11 | 1.00 ± 0.01 | 3.04 ± 0.11 | 1.03 ± 0.02 | 2.95 ± 0.10 | 1.01 ± 0.01 | |
| Glutamate | 1 | 15.60 ± 0.01 | 19.03 ± 0.01 | 18.74 ± 0.00 | 19.14 ± 0.00 | 9.92 ± 0.00 | 22.76 ± 0.00 | 9.67 ± 0.00 | 4.73 ± 0.00 | 13.49 ± 0.01 | 1.61 ± 0.00 | 19.09 ± 0.00 | 4.61 ± 0.00 | 2.25 ± 0.00 | 2.87 ± 0.00 | 2.48 ± 0.00 |
| 2 | 13.95 ± 0.00 | 19.13 ± 0.00 | 12.31 ± 0.00 | 15.54 ± 0.01 | 11.24 ± 0.00 | 18.13 ± 0.00 | 13.35 ± 0.00 | 5.23 ± 0.00 | 18.78 ± 0.00 | 1.83 ± 0.00 | 16.62 ± 0.00 | 5.16 ± 0.00 | 3.26 ± 0.00 | 14.23 ± 0.00 | 3.17 ± 0.00 | |
| 3 | 21.19 ± 0.00 | 21.00 ± 0.00 | 12.09 ± 0.00 | 21.15 ± 0.00 | 14.94 ± 0.00 | 27.49 ± 0.00 | 13.85 ± 0.00 | 12.62 ± 0.00 | 18.22 ± 0.00 | 4.38 ± 0.01 | 5.40 ± 0.00 | 6.07 ± 0.00 | 9.11 ± 0.00 | 5.41 ± 0.00 | 4.01 ± 0.00 | |
| Glycero-phospho-choline | 1 | ** | 71.79 ± 0.004 | 25.41 ± 0.005 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| 2 | ** | 56.07 ± 0.003 | 28.42 ± 0.001 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| 3 | ** | 82.68 ± 0.000 | 34.01 ± 0.000 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Glycine | 1 | 12.92 ± 0.00 | 15.00 ± 0.00 | 3.88 ± 0.00 | 14.25 ± 0.00 | 3.27 ± 0.00 | 13.46 ± 0.01 | 3.78 ± 0.01 | 11.09 ± 0.01 | 5.47 ± 0.00 | 11.48 ± 0.00 | 2.98 ± 0.00 | 11.14 ± 0.00 | 3.38 ± 0.00 | 15.31 ± 0.00 | 3.97 ± 0.00 |
| 2 | 11.94 ± 0.00 | 12.49 ± 0.00 | 3.80 ± 0.00 | 11.18 ± 0.00 | 4.86 ± 0.00 | 14.36 ± 0.00 | 4.31 ± 0.00 | 10.75 ± 0.01 | 4.72 ± 0.00 | 11.90 ± 0.00 | 3.82 ± 0.00 | 16.00 ± 0.00 | 1.85 ± 0.00 | 25.42 ± 0.00 | 4.23 ± 0.00 | |
| 3 | 14.42 ± 0.00 | 16.17 ± 0.01 | 4.79 ± 0.00 | 16.06 ± 0.02 | 6.57 ± 0.00 | 16.40 ± 0.01 | 2.64 ± 0.00 | 13.95 ± 0.00 | 4.62 ± 0.00 | 13.94 ± 0.00 | 3.97 ± 0.00 | 16.31 ± 0.00 | 5.02 ± 0.00 | 21.84 ± 0.00 | 3.62 ± 0.00 | |
| Hypoxanthine | 1 | 7.16 ± 0.00 | 20.00 ± 0.00 | 6.57 ± 0.00 | 15.00 ± 0.00 | 6.14 ± 0.00 | 20.54 ± 0.00 | 5.98 ± 0.00 | 20.82 ± 0.00 | 6.70 ± 0.00 | 34.04 ± 0.00 | 8.26 ± 0.00 | 49.65 ± 0.00 | 9.64 ± 0.00 | 62.02 ± 0.00 | 7.42 ± 0.00 |
| 2 | 7.62 ± 0.00 | 18.52 ± 0.00 | 7.55 ± 0.00 | 10.32 ± 0.00 | 5.73 ± 0.00 | 13.68 ± 0.00 | 5.28 ± 0.00 | 15.00 ± 0.00 | 8.86 ± 0.00 | 55.79 ± 0.01 | 8.04 ± 0.00 | 76.23 ± 0.00 | 9.71 ± 0.00 | 53.53 ± 0.00 | 17.47 ± 0.00 | |
| 3 | 7.72 ± 0.00 | 22.87 ± 0.00 | 5.92 ± 0.00 | 17.03 ± 0.00 | 7.60 ± 0.00 | 18.61 ± 0.00 | 4.85 ± 0.00 | 20.17 ± 0.00 | 6.45 ± 0.00 | 32.88 ± 0.00 | 4.24 ± 0.00 | 63.48 ± 0.02 | 10.65 ± 0.00 | 62.30 ± 0.00 | 12.48 ± 0.00 | |
| Inosine | 1 | 110.71 ± 0.00 | 117.85 ± 0.00 | 81.51 ± 0.01 | 181.74 ± 0.00 | 89.96 ± 0.00 | 177.02 ± 0.00 | 62.72 ± 0.00 | 128.49 ± 0.00 | 79.13 ± 0.01 | 115.07 ± 0.00 | 59.66 ± 0.00 | 50.35 ± 0.00 | 28.15 ± 0.00 | 10.27 ± 0.00 | 41.06 ± 0.00 |
| 2 | 128.95 ± 0.00 | 136.13 ± 0.00 | 69.73 ± 0.00 | 159.97 ± 0.00 | 103.49 ± 0.01 | 184.19 ± 0.00 | 72.14 ± 0.00 | 159.10 ± 0.00 | 87.23 ± 0.00 | 65.76 ± 0.00 | 62.77 ± 0.00 | 21.41 ± 0.00 | 22.00 ± 0.00 | 15.50 ± 0.00 | 16.20 ± 0.00 | |
| 3 | 141.51 ± 0.00 | 140.81 ± 0.00 | 88.91 ± 0.00 | 193.67 ± 0.01 | 109.70 ± 0.00 | 195.66 ± 0.00 | 61.00 ± 0.00 | 171.78 ± 0.01 | 60.51 ± 0.00 | 80.21 ± 0.00 | 34.09 ± 0.00 | 30.13 ± 0.00 | 32.20 ± 0.00 | 12.57 ± 0.00 | 12.54 ± 0.00 | |
| IMP | 1 | 102.70 ± 0.00 | 132.37 ± 0.00 | 8.43 ± 0.00 | 8.07 ± 0.00 | 20.04 ± 0.00 | 7.43 ± 0.00 | 4.13 ± 0.00 | 7.77 ± 0.00 | 0.00 | 7.72 ± 0.00 | 0.00 | 6.11 ± 0.00 | 0.00 | 2.50 ± 0.00 | 0.00 |
| 2 | 103.38 ± 0.00 | 58.67 ± 0.00 | 19.10 ± 0.00 | 34.40 ± 0.00 | 7.13 ± 0.00 | 5.56 ± 0.00 | 1.91 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 3 | 120.59 ± 0.01 | 116.16 ± 0.00 | 24.71 ± 0.00 | 47.29 ± 0.00 | 8.39 ± 0.00 | 6.28 ± 0.00 | 0.00 | 7.03 ± 0.00 | 0.00 | 8.37 ± 0.00 | 0.00 | 4.66 ± 0.00 | 0.00 | 3.00 ± 0.00 | 0.00 | |
| Lactate | 1 | 576.51 ± 0.01 | 613.29 ± 0.01 | 219.11 ± 0.01 | 652.26 ± 0.01 | 250.87 ± 0.01 | 643.03 ± 0.01 | 182.97 ± 0.00 | 468.45 ± 0.01 | 225.64 ± 0.03 | 527.62 ± 0.00 | 179.59 ± 0.01 | 304.69 ± 0.02 | 116.81 ± 0.01 | 360.98 ± 0.01 | 146.79 ± 0.00 |
| 2 | 588.61 ± 0.00 | 506.47 ± 0.00 | 180.55 ± 0.00 | 545.75 ± 0.00 | 253.77 ± 0.00 | 568.22 ± 0.02 | 178.19 ± 0.00 | 485.83 ± 0.02 | 221.04 ± 0.01 | 454.54 ± 0.02 | 167.49 ± 0.00 | 365.82 ± 0.01 | 102.09 ± 0.01 | 159.40 ± 0.01 | 143.21 ± 0.01 | |
| 3 | 633.48 ± 0.01 | 578.85 ± 0.00 | 207.80 ± 0.00 | 674.83 ± 0.00 | 278.22 ± 0.01 | 593.79 ± 0.03 | 147.24 ± 0.01 | 530.04 ± 0.00 | 153.36 ± 0.01 | 218.87 ± 0.00 | 102.29 ± 0.02 | 163.72 ± 0.01 | 137.20 ± 0.01 | 280.87 ± 0.00 | 48.65 ± 0.00 | |
| Mannitol | 1 | 0.00 | 0.00 | 303.00 ± 0.01 | 0.00 | 142.25 ± 0.01 | 0.00 | 147.36 ± 0.01 | 0.00 | 223.74 ± 0.01 | 0.00 | 642.18 ± 0.04 | 0.00 | 112.66 ± 0.01 | 0.00 | 144.45 ± 0.03 |
| 2 | 0.00 | 0.00 | 257.48± 0.02 | 0.00 | 168.25 ± 0.01 | 0.00 | 229.00 ± 0.01 | 0.00 | 513.98 ± 0.04 | 0.00 | 514.37 ± 0.02 | 0.00 | 382.38 ± 0.02 | 0.00 | 236.53 ± 0.01 | |
| 3 | 0.00 | 0.00 | 125.65 ± 0.00 | 0.00 | 219.55 ± 0.01 | 0.00 | 366.80 ± 0.01 | 0.00 | 459.45 ± 0.02 | 0.00 | 172.09 ± 0.01 | 0.00 | 530.34 ± 0.01 | 0.00 | 257.82 ± 0.01 | |
| Nicotinate | 1 | 6.89 ± 0.00 | 7.14 ± 0.00 | 2.79 ± 0.00 | 7.93 ± 0.00 | 3.26 ± 0.00 | 8.57 ± 0.00 | 2.46 ± 0.00 | 7.16 ± 0.00 | 3.87 ± 0.00 | 7.69 ± 0.00 | 2.52 ± 0.00 | 7.12 ± 0.00 | 2.05 ± 0.00 | 7.14 ± 0.00 | 2.82 ± 0.00 |
| 2 | 7.26 ± 0.00 | 6.32 ± 0.00 | 2.75 ± 0.00 | 7.03 ± 0.00 | 3.87 ± 0.00 | 8.34 ± 0.00 | 2.80 ± 0.00 | 7.46 ± 0.00 | 3.69 ± 0.00 | 7.98 ± 0.00 | 2.87 ± 0.00 | 7.89 ± 0.00 | 1.50 ± 0.00 | 6.82 ± 0.00 | 2.80 ± 0.00 | |
| 3 | 7.93 ± 0.00 | 8.02 ± 0.00 | 3.05 ± 0.00 | 8.03 ± 0.00 | 3.55 ± 0.00 | 7.82 ± 0.00 | 2.31 ± 0.00 | 8.02 ± 0.00 | 1.94 ± 0.00 | 7.84 ± 0.00 | 2.45 ± 0.00 | 6.32 ± 0.00 | 2.10 ± 0.00 | 6.94 ± 0.00 | 1.74 ± 0.00 | |
| Phenylalanine | 1 | 3.63 ± 0.00 | 5.46 ± 0.00 | 2.34 ± 0.00 | 4.39 ± 0.00 | 3.63 ± 0.00 | 5.81 ± 0.00 | 3.38 ± 0.00 | 5.53 ± 0.00 | 5.38 ± 0.00 | 6.40 ± 0.00 | 2.99 ± 0.00 | 11.67 ± 0.00 | 4.24 ± 0.00 | 11.34 ± 0.00 | 6.02 ± 0.00 |
| 2 | 2.98 ± 0.00 | 6.03 ± 0.01 | 3.01 ± 0.00 | 5.00 ± 0.00 | 3.01 ± 0.00 | 6.59 ± 0.00 | 3.51 ± 0.00 | 6.15 ± 0.00 | 4.53 ± 0.00 | 8.81 ± 0.00 | 3.69 ± 0.00 | 17.10 ± 0.00 | 2.08 ± 0.00 | 27.77 ± 0.00 | 4.42 ± 0.00 | |
| 3 | 4.66 ± 0.00 | 5.23 ± 0.00 | 3.29 ± 0.00 | 5.23 ± 0.00 | 3.57 ± 0.00 | 6.45 ± 0.00 | 2.46 ± 0.00 | 5.54 ± 0.00 | 3.82 ± 0.00 | 7.21 ± 0.00 | 2.80 ± 0.00 | 13.36 ± 0.00 | 3.89 ± 0.00 | 18.58 ± 0.00 | 4.92 ± 0.00 | |
| Succinate | 1 | 1.37 ± 0.00 | 0.18 ± 0.00 | 8.51 ± 0.00 | 0.00 | 3.11 ± 0.00 | 0.00 | 3.70 ± 0.00 | 0.17 ± 0.00 | 2.49 ± 0.00 | 1.47 ± 0.00 | 1.86 ± 0.00 | 4.41 ± 0.00 | 0.34 ± 0.00 | 21.26 ± 0.00 | 0.92 ± 0.00 |
| 2 | 0.86 ± 0.00 | 0.22 ± 0.00 | 4.94 ± 0.00 | 0.00 | 3.17 ± 0.00 | 0.00 | 4.60 ± 0.00 | 0.08 ± 0.00 | 2.90 ± 0.00 | 5.64 ± 0.00 | 3.43 ± 0.00 | 19.07 ± 0.00 | 1.39 ± 0.00 | 7.68 ± 0.00 | 3.82 ± 0.00 | |
| 3 | 0.36 ± 0.00 | 0.00 | 5.48 ± 0.00 | 0.00 | 4.86 ± 0.00 | 0.00 | 4.44 ± 0.00 | 0.11 ± 0.00 | 5.46 ± 0.00 | 0.29 ± 0.00 | 0.87 ± 0.00 | 1.98 ± 0.00 | 4.99 ± 0.00 | 14.35 ± 0.00 | 1.29 ± 0.00 | |
| TMA | 1 | 0.52 ± 0.00 | 0.15 ± 0.00 | 0.22 ± 0.00 | 0.36 ± 0.00 | 0.11 ± 0.00 | 0.95 ± 0.00 | 1.17 ± 0.00 | 1.90 ± 0.00 | 0.23 ± 0.00 | 14.83 ± 0.00 | 0.37 ± 0.00 | 17.18 ± 0.01 | 0.61 ± 0.00 | 40.75 ± 0.01 | 0.72 ± 0.00 |
| 2 | 0.08 ± 0.00 | 0.24 ± 0.00 | 0.06 ± 0.00 | 0.29 ± 0.00 | 0.09 ± 0.00 | 0.28 ± 0.00 | 0.05 ± 0.00 | 1.22 ± 0.00 | 0.10 ± 0.00 | 26.85 ± 0.01 | 0.30 ± 0.00 | 38.96 ± 0.00 | 0.40 ± 0.00 | 23.07 ± 0.00 | 2.39 ± 0.00 | |
| 3 | 0.26 ± 0.00 | 0.20 ± 0.00 | 0.13 ± 0.00 | 0.47 ± 0.00 | 0.28 ± 0.00 | 0.90 ± 0.00 | 0.13 ± 0.00 | 1.01 ± 0.00 | 0.12 ± 0.00 | 2.70 ± 0.00 | 0.34 ± 0.00 | 14.82 ± 0.00 | 0.81 ± 0.00 | 34.35 ± 0.00 | 1.72 ± 0.00 | |
| TMAO | 1 | 50.85 ± 0.00 | 51.30 ± 0.02 | 21.25 ± 0.01 | 44.16 ± 0.02 | 18.08 ± 0.00 | 55.81 ± 0.04 | 12.02 ± 0.00 | 54.43 ± 0.01 | 21.28 ± 0.00 | 27.32 ± 0.01 | 14.38 ± 0.01 | 24.97 ± 0.01 | 19.72 ± 0.01 | 0.00 | 17.87 ± 0.01 |
| 2 | 53.33 ± 0.02 | 66.23 ± 0.01 | 21.56 ± 0.00 | 59.66 ± 0.01 | 23.96 ± 0.01 | 55.07 ± 0.02 | 15.66 ± 0.01 | 58.93 ± 0.00 | 22.83 ± 0.00 | 17.42 ± 0.01 | 19.00 ± 0.01 | 3.04 ± 0.00 | 14.68 ± 0.01 | 28.60 ± 0.01 | 16.46 ± 0.02 | |
| 3 | 65.95 ± 0.02 | 49.95 ± 0.00 | 18.93 ± 0.02 | 55.08 ± 0.00 | 21.84 ± 0.01 | 55.27 ± 0.03 | 13.99 ± 0.01 | 53.69 ± 0.01 | 11.97 ± 0.00 | 53.36 ± 0.02 | 19.77 ± 0.01 | 29.86 ± 0.03 | 15.13 ± 0.01 | 3.63 ± 0.00 | 13.61 ± 0.01 | |
| Tyrosine | 1 | 6.41 ± 0.00 | 8.26 ± 0.00 | 2.65 ± 0.00 | 6.88 ± 0.00 | 5.24 ± 0.00 | 8.01 ± 0.00 | 3.77 ± 0.00 | 7.35 ± 0.00 | 6.04 ± 0.00 | 8.51 ± 0.00 | 2.81 ± 0.00 | 11.75 ± 0.00 | 4.76 ± 0.00 | 12.82 ± 0.00 | 6.32 ± 0.00 |
| 2 | 6.64 ± 0.00 | 6.46 ± 0.00 | 3.95 ± 0.00 | 6.93 ± 0.00 | 4.11 ± 0.00 | 8.87 ± 0.00 | 4.13 ± 0.00 | 7.50 ± 0.00 | 4.69 ± 0.00 | 10.50 ± 0.00 | 3.85 ± 0.00 | 18.82 ± 0.01 | 2.15 ± 0.00 | 30.84 ± 0.00 | 4.30 ± 0.00 | |
| 3 | 7.98 ± 0.00 | 8.44 ± 0.00 | 4.80 ± 0.00 | 10.25 ± 0.00 | 5.97 ± 0.00 | 10.00 ± 0.00 | 3.74 ± 0.00 | 8.57 ± 0.00 | 4.12 ± 0.00 | 10.24 ± 0.00 | 3.73 ± 0.00 | 11.19 ± 0.00 | 4.24 ± 0.00 | 8.29 ± 0.00 | 4.92 ± 0.00 | |
| Valine | 1 | 8.19 ± 0.00 | 9.76 ± 0.00 | 5.07 ± 0.00 | 9.39 ± 0.00 | 5.16 ± 0.00 | 12.16 ± 0.00 | 4.73 ± 0.00 | 8.95 ± 0.00 | 7.84 ± 0.00 | 9.98 ± 0.00 | 7.03 ± 0.00 | 15.58 ± 0.00 | 6.86 ± 0.00 | 14.50 ± 0.00 | 8.59 ± 0.01 |
| 2 | 9.50 ± 0.00 | 10.32 ± 0.00 | 5.34 ± 0.00 | 9.83 ± 0.00 | 6.01 ± 0.00 | 11.87 ± 0.00 | 5.41 ± 0.00 | 9.89 ± 0.00 | 7.21 ± 0.00 | 13.29 ± 0.00 | 6.08 ± 0.00 | 23.15 ± 0.00 | 4.04 ± 0.00 | 33.22 ± 0.01 | 8.51 ± 0.00 | |
| 3 | 11.17 ± 0.01 | 10.94 ± 0.00 | 5.62 ± 0.00 | 12.37 ± 0.00 | 7.57 ± 0.00 | 12.11 ± 0.00 | 5.12 ± 0.00 | 11.16 ± 0.00 | 6.31 ± 0.00 | 12.79 ± 0.00 | 6.38 ± 0.00 | 18.18 ± 0.00 | 7.07 ± 0.00 | 22.43 ± 0.00 | 8.71 ± 0.00 | |
| Experiment 2. (Evaluation) | ||||||||||||||||
| Compound | 7 | 9 | 11 | 14 | 17 | 19 | 21 | |||||||||
| RS-2 | RS-2 | STS-2 | RS-2 | STS-2 | RS-2 | STS-2 | RS-2 | STS-2 | RS-2 | STS-2 | RS-2 | STS-2 | ||||
| 2,3-butanediol | 0.12 ± 0.00 | 1.27 ± 0.00 | 4.72 ± 0.00 | 96.78 ± 0.00 | 57.37 ± 0.00 | 119.28 ± 0.01 | 111.50 ± 0.01 | 131.18 ± 0.01 | 95.74 ± 0.00 | 124.69 ± 0.01 | 74.89 ± 0.02 | 124.23 ± 0.00 | 90.04 ± 0.01 | |||
| Acetoin | 0.00 | 4.47 ± 0.00 | 7.78 ± 0.00 | 24.47 ± 0.00 | 25.18 ± 0.00 | 17.39 ± 0.00 | 37.21 ± 0.01 | 27.14 ± 0.01 | 45.75 ± 0.02 | 25.78 ± 0.00 | 33.76 ± 0.00 | 22.95 ± 0.00 | 44.56 ± 0.01 | |||
| Alanine | 65.42 ± 0.00 | 71.11 ± 0.01 | 76.36 ± 0.00 | 88.59 ± 0.00 | 76.97 ± 0.01 | 86.78 ± 0.00 | 84.91 ± 0.00 | 82.55 ± 0.01 | 83.95 ± 0.00 | 85.54 ± 0.00 | 87.61 ± 0.01 | 87.10 ± 0.01 | 84.53 ± 0.01 | |||
| Anserine | 580.57 ± 0.02 | 488.55 ± 0.01 | 556.42 ± 0.01 | 603.14 ± 0.00 | 477.29 ± 0.00 | 679.32 ± 0.01 | 540.87 ± 0.01 | 596.26 ± 0.01 | 433.81 ± 0.01 | 618.26 ± 0.00 | 423.81 ± 0.00 | 646.31 ± 0.01 | 378.07 ± 0.00 | |||
| β-Alanine | 19.78 ± 0.00 | 18.61 ± 0.00 | 19.46 ± 0.00 | 24.97 ± 0.00 | 18.80 ± 0.00 | 27.23 ± 0.00 | 24.24 ± 0.00 | 24.81 ± 0.00 | 22.29 ± 0.00 | 24.12 ± 0.01 | 22.62 ± 0.00 | 25.81 ± 0.01 | 25.63 ± 0.00 | |||
| Betaine | 12.04 ± 0.01 | 14.61 ± 0.00 | 11.34 ± 0.00 | 13.48 ± 0.00 | 8.47 ± 0.00 | 13.30 ± 0.00 | 9.96 ± 0.00 | 13.53 ± 0.00 | 8.21 ± 0.00 | 11.54 ± 0.00 | 7.50 ± 0.00 | 12.72 ± 0.00 | 10.76 ± 0.00 | |||
| Creatine (mmol/100 g) | 3.93 ± 0.02 | 3.64 ± 0.00 | 3.27 ± 0.04 | 4.06 ± 0.03 | 2.80 ± 0.01 | 4.27 ± 0.02 | 3.49 ± 0.01 | 3.91 ± 0.03 | 2.90 ± 0.01 | 3.96 ± 0.03 | 2.80 ± 0.02 | 3.99 ± 0.02 | 2.89 ± 0.03 | |||
| Ethanol | 2.64 ± 0.00 | 1.75 ± 0.00 | 2.03 ± 0.00 | 15.73 ± 0.01 | 3.83 ± 0.01 | 20.18 ± 0.00 | 5.20 ± 0.00 | 15.46 ± 0.00 | 6.99 ±0.01 | 20.36 ± 0.00 | 9.59 ± 0.00 | 21.87 ± 0.00 | 8.75 ± 0.00 | |||
| Formate | 0.26 ± 0.00 | 0.65 ± 0.00 | 1.50 ± 0.00 | 12.87 ± 0.00 | 23.55 ± 0.00 | 0.82 ± 0.00 | 28.67 ± 0.00 | 0.81 ± 0.00 | 19.47 ± 0.00 | 0.50 ± 0.00 | 23.84 ± 0.00 | 0.95 ± 0.00 | 4.07 ± 0.00 | |||
| Fumarate | 0.43 ± 0.00 | * | * | 0.34 ± 0.00 | * | 0.47 ± 0.00 | * | 0.49 ± 0.00 | * | 0.50 ± 0.00 | * | * | * | |||
| Glutamate | 15.01 ± 0.00 | 17.03 ± 0.00 | 17.02 ± 0.00 | 18.99 ± 0.00 | 17.15 ± 0.00 | 10.47 ± 0.00 | 14.58 ± 0.00 | 7.00 ± 0.00 | 13.65 ± 0.00 | 6.84 ± 0.00 | 7.75 ± 0.00 | 6.15 ± 0.00 | 10.77 ± 0.00 | |||
| Glycine | 47.41 ± 0.00 | 45.61 ± 0.01 | 38.73 ± 0.01 | 52.61 ± 0.02 | 33.37 ± 0.01 | 50.40 ± 0.01 | 40.72 ± 0.01 | 52.27 ± 0.02 | 36.42 ± 0.00 | 54.39 ± 0.01 | 34.79 ± 0.02 | 55.81 ±0.03 | 44.09 ± 0.02 | |||
| Histamine | 0.00 | 0.00 | 0.00 | 31.99 ± 0.01 | 22.07 ± 0.01 | 41.32 ± 0.00 | 33.61 ± 0.00 | 32.18 ± 0.00 | 27.47 ± 0.00 | 32.41 ± 0.01 | 29.47 ± 0.01 | 34.10 ± 0.00 | 30.07 ± 0.00 | |||
| Hypoxanthine | 17.23 ± 0.00 | 26.54 ± 0.00 | 28.76 ± 0.00 | 88.21 ± 0.00 | 45.95 ± 0.00 | 93.83 ± 0.01 | 42.26 ± 0.00 | 80.06 ± 0.00 | 21.36 ± 0.00 | 82.82 ± 0.00 | 26.51 ± 0.00 | 85.22 ± 0.00 | 28.13 ± 0.01 | |||
| Inosine | 116.04 ± 0.00 | 121.68 ± 0.00 | 116.28 ± 0.00 | 8.05 ± 0.00 | 6.05 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| IMP | 70.35 ± 0.00 | 1.88 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Mannitol | 0.00 | 0.00 | 52.41 ± 0.00 | 0.00 | 151.47 ± 0.01 | 0.00 | 114.94 ± 0.01 | 0.00 | 139.94 ± 0.01 | 0.00 | 185.30 ± 0.00 | 0.00 | 94.09 ± 0.00 | |||
| Nicotinate | 5.69 ± 0.00 | 4.99 ± 0.00 | 5.80 ± 0.00 | 6.98 ± 0.00 | 5.17 ± 0.00 | 8.18 ± 0.00 | 6.68 ± 0.00 | 7.03 ± 0.00 | 5.37 ± 0.00 | 6.91 ± 0.00 | 5.17 ± 0.00 | 7.82 ± 0.00 | 5.37 ± 0.00 | |||
| Putrescine | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.74 ± 0.00 | 3.38 ± 0.00 | 7.22 ± 0.00 | 3.24 ± 0.01 | 8.43 ± 0.00 | 6.60 ± 0.00 | 9.04 ± 0.00 | 5.53 ± 0.00 | |||
| Succinate | 0.21 ± 0.00 | 0.13 ± 0.00 | 0.61 ± 0.00 | 3.10 ± 0.00 | 4.09 ± 0.00 | 9.46 ± 0.01 | 9.68 ± 0.00 | 10.09 ± 0.01 | 17.19 ± 0.01 | 14.81 ± 0.01 | 20.24 ± 0.00 | 22.49 ± 0.00 | 21.96 ± 0.00 | |||
| TMA | 0.28 ± 0.00 | 6.91 ± 0.00 | 8.57 ± 0.00 | 22.38 ± 0.02 | 20.14 ± 0.01 | 32.75 ± 0.01 | 22.69 ± 0.01 | 35.84 ± 0.02 | 24.96 ± 0.01 | 39.85 ± 0.01 | 24.25 ± 0.00 | 46.36 ± 0.01 | 27.69 ± 0.00 | |||
| TMAO | 17.43 ± 0.01 | 14.08 ± 0.01 | 9.07 ± 0.01 | 0.00 | 1.49 ± 0.00 | 0.00 | 0.00 | 0.46 ± 0.01 | 0.21 ± 0.01 | 0.08 ± 0.00 | 0.63 ± 0.01 | 0.00 | 0.40 ± 0.00 | |||
| Tyramine | 0.00 | 0.00 | 0.00 | 2.34 ± 0.00 | 2.27 ± 0.00 | 3.00 ± 0.00 | 3.39 ± 0.00 | 2.68 ± 0.00 | 3.61 ± 0.00 | 3.72 ± 0.00 | 5.00 ± 0.00 | 4.36 ± 0.00 | 3.51 ± 0.00 | |||
| Tyrosine | 7.00 ± 0.00 | 5.82 ± 0.00 | 7.60 ± 0.00 | 0.95 ± 0.00 | 1.36 ± 0.00 | 0.75 ± 0.00 | 0.84 ± 0.00 | 1.32 ± 0.00 | 1.11 ± 0.00 | 1.01 ± 0.00 | 1.14 ± 0.00 | 1.07 ± 0.00 | 1.03 ± 0.00 | |||
| Uracil | 0.67 ± 0.00 | 0.58 ± 0.00 | 0.70 ± 0.00 | 0.00 | 0.00 | 0.00 | 0.53 ± 0.00 | 0.36 ± 0.00 | 1.14 ± 0.00 | 0.82 ± 0.00 | 1.45 ± 0.00 | 1.13 ± 0.00 | 1.18 ± 0.00 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkholt, E.M.; Dikiy, A.; Shumilina, E. Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment. Foods 2019, 8, 625. https://doi.org/10.3390/foods8120625
Kirkholt EM, Dikiy A, Shumilina E. Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment. Foods. 2019; 8(12):625. https://doi.org/10.3390/foods8120625
Chicago/Turabian StyleKirkholt, Even Moen, Alexander Dikiy, and Elena Shumilina. 2019. "Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment" Foods 8, no. 12: 625. https://doi.org/10.3390/foods8120625
APA StyleKirkholt, E. M., Dikiy, A., & Shumilina, E. (2019). Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment. Foods, 8(12), 625. https://doi.org/10.3390/foods8120625






