Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Noroviruses
2.2. Spiking of NoV GI and GII onto Fresh Produce
2.3. Chemical Disinfection Treatment
2.4. Sample Processing for Virus Extraction and Quantification
2.5. Data Analysis
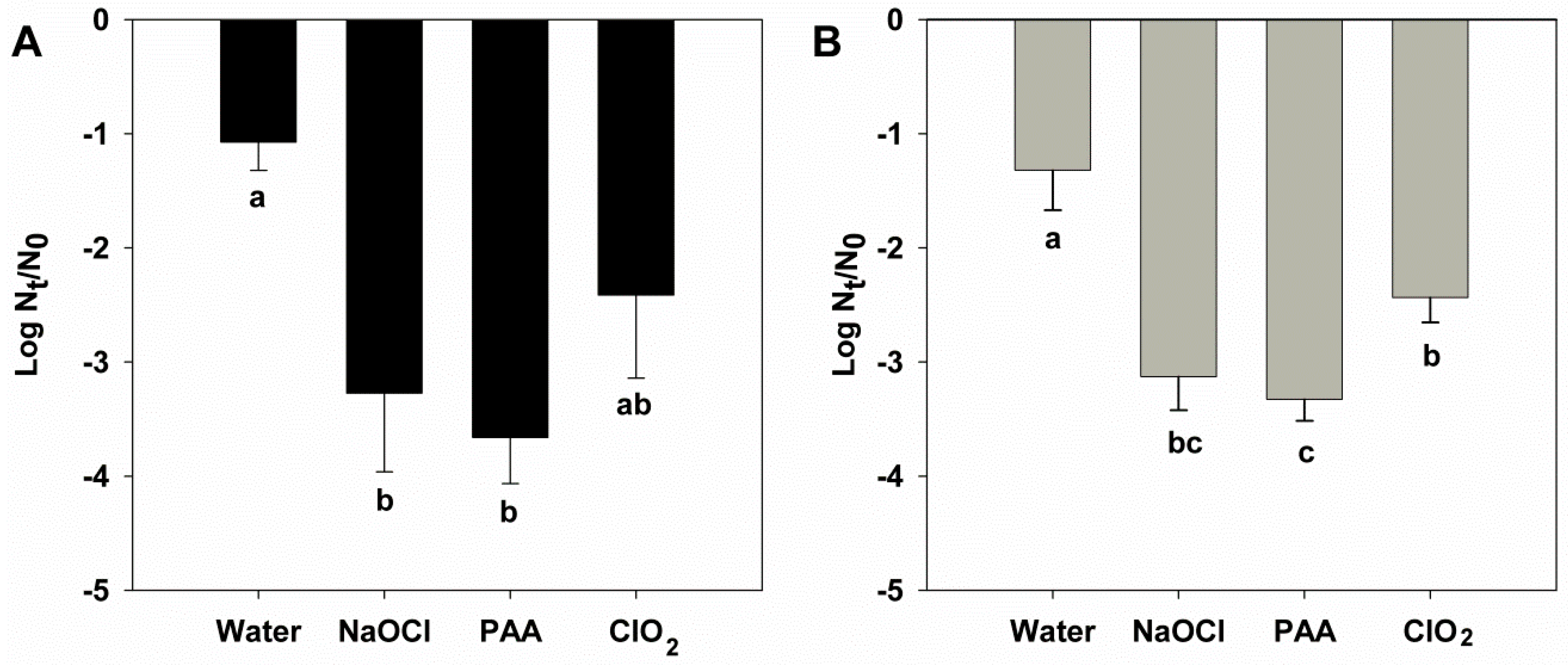
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; van Beek, J.; Koopmans, M.P.G. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, 4634. [Google Scholar] [CrossRef]
- ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar] [CrossRef]
- Li, M.; Baker, C.A.; Danyluk, M.D.; Belanger, P.; Boelaert, F.; Cressey, P.; Gheorghe, M.; Polkinghorne, B.; Toyofuku, H.; Havelaar, A.H. Identification of Biological Hazards in Produce Consumed in Industrialized Countries: A Review. J. Food Prot. 2018, 81, 1171–1186. [Google Scholar] [CrossRef]
- Aron, J.H.; Valerie, G.E.; Amy Lehman, E.; Gould, L.H.; Ben, A.L.; Umesh, D.P. Epidemiology of Foodborne Norovirus Outbreaks, United States, 2001–2008. Emerg. Infect. Dis. 2012, 18, 1566. [Google Scholar] [CrossRef]
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Söderberg, K.; Vasickova, P.; Bouwknegt, M.; Rutjes, S.; Willems, K.; Moloney, R.; de Roda Husman, A.M.; et al. Virological Quality of Irrigation Water in Leafy Green Vegetables and Berry Fruits Production Chains. Food Environ. Virol. 2017, 9, 72–78. [Google Scholar] [CrossRef]
- Cook, N.; Knight, A.; Richards, G.P. Persistence and Elimination of Human Norovirus in Food and on Food Contact Surfaces: A Critical Review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef]
- ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J. 2012, 10, 2597. [Google Scholar]
- Hall, A.J.; Wikswo, M.E.; Pringle, K.; Gould, L.H.; Parashar, U.D. Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC. Vital signs: Foodborne norovirus outbreaks—United States, 2009-2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 491–495. [Google Scholar] [PubMed]
- Baert, L.; Mattison, K.; Loisy-Hamon, F.; Harlow, J.; Martyres, A.; Lebeau, B.; Stals, A.; Van Coillie, E.; Herman, L.; Uyttendaele, M. Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? Int. J. Food Microbiol. 2011, 151, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Guix, S.; Pinto, R.M.; Bosch, A. Final Consumer Options to Control and Prevent Foodborne Norovirus Infections. Viruses 2019, 11, 333. [Google Scholar] [CrossRef]
- Cook, N.; Williams, L.; D’Agostino, M. Prevalence of Norovirus in produce sold at retail in the United Kingdom. Food Microbiol. 2019, 79, 85–89. [Google Scholar] [CrossRef]
- Fraisse, A.; Temmam, S.; Deboosere, N.; Guillier, L.; Delobel, A.; Maris, P.; Vialette, M.; Morin, T.; Perelle, S. Comparison of chlorine and peroxyacetic-based disinfectant to inactivate Feline calicivirus, Murine norovirus and Hepatitis A virus on lettuce. Int. J. Food Microbiol. 2011, 151, 98–104. [Google Scholar] [CrossRef]
- Girard, M.; Mattison, K.; Fliss, I.; Jean, J. Efficacy of oxidizing disinfectants at inactivating murine norovirus on ready-to-eat foods. Int. J. Food Microbiol. 2016, 219, 7–11. [Google Scholar] [CrossRef]
- Dunkin, N.; Weng, S.; Jacangelo, J.G.; Schwab, K.J. Inactivation of Human Norovirus Genogroups I and II and Surrogates by Free Chlorine in Postharvest Leafy Green Wash Water. Appl. Environ. Microbiol. 2017, 83, e01457-17. [Google Scholar] [CrossRef]
- Dunkin, N.; Coulter, C.; Weng, S.; Jacangelo, J.G.; Schwab, K.J. Effects of pH Variability on Peracetic Acid Reduction of Human Norovirus GI, GII RNA, and Infectivity Plus RNA Reduction of Selected Surrogates. Food Environ. Virol. 2018. [Google Scholar] [CrossRef]
- Randazzo, W.; López-Gálvez, F.; Allende, A.; Aznar, R.; Sánchez, G. Evaluation of viability PCR performance for assessing norovirus infectivity in fresh-cut vegetables and irrigation water. Int. J. Food Microbiol. 2016, 229, 1–6. [Google Scholar] [CrossRef]
- Manuel, C.S.; Moore, M.D.; Jaykus, L.-A. Predicting human norovirus infectivity—Recent advances and continued challenges. Food Microbiol. 2018, 76, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sabria, A.; Pinto, R.M.; Bosch, A.; Bartolome, R.; Cornejo, T.; Torner, N.; Martinez, A.; de Simon, M.; Dominguez, A.; Guix, S. Molecular and clinical epidemiology of norovirus outbreaks in Spain during the emergence of GII.4 2012 variant. J. Clin. Virol. 2014, 60, 96–104. [Google Scholar] [CrossRef] [PubMed]
- ISO15216-1:2017. Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Lowther, J.A.; Bosch, A.; Butot, S.; Ollivier, J.; Mäde, D.; Rutjes, S.A.; Hardouin, G.; Lombard, B.; In’t Veld, P.; Leclercq, A. Validation of EN ISO method 15216—Part 1—Quantification of hepatitis A virus and norovirus in food matrices. Int. J. Food Microbiol. 2019, 288, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Fuster, N.; Pintó, R.M.; Fuentes, C.; Beguiristain, N.; Bosch, A.; Guix, S. Propidium monoazide RTqPCR assays for the assessment of hepatitis A inactivation and for a better estimation of the health risk of contaminated waters. Water Res. 2016, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Banach, J.L.; Sampers, I.; Van Haute, S.; van der Fels-Klerx, H.J. Effect of Disinfectants on Preventing the Cross-Contamination of Pathogens in Fresh Produce Washing Water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, S.Y. Review: Comparison of the effectiveness of decontaminating strategies for fresh fruits and vegetables and related limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 3189–3208. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Randazzo, W.; Vásquez, A.; Sánchez, G.; Decol, L.T.; Aznar, R.; Gil, M.I.; Allende, A. Irrigating Lettuce with Wastewater Effluent: Does Disinfection with Chlorine Dioxide Inactivate Viruses? J. Environ. Qual. 2018, 47, 1139–1145. [Google Scholar] [CrossRef]
- Parshionikar, S.; Laseke, I.; Fout, G.S. Use of Propidium Monoazide in Reverse Transcriptase PCR To Distinguish between Infectious and Noninfectious Enteric Viruses in Water Samples. Appl. Environ. Microbiol. 2010, 76, 4318–4326. [Google Scholar] [CrossRef]
- Leifels, M.; Jurzik, L.; Wilhelm, M.; Hamza, I.A. Use of ethidium monoazide and propidium monoazide to determine viral infectivity upon inactivation by heat, UV-exposure and chlorine. Int. J. Hyg. Environ. Health 2015. [Google Scholar] [CrossRef]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.-L.; Qu, L.; et al. Replication of human noroviruses in stem cell–derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinje, J. Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Butot, S.; Putallaz, T.; Sanchez, G. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int. J. Food Microbiol. 2008, 126, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Lee, J.S.; Shin, M.H.; Lee, S.H.; Hwang, I.G. Effect of wash treatments on reducing human norovirus on iceberg lettuce and perilla leaf. J. Food Prot. 2011, 74, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; Vandekinderen, I.; Devlieghere, F.; Van Coillie, E.; Debevere, J.; Uyttendaele, M. Efficacy of sodium hypochlorite and peroxyacetic acid to reduce murine norovirus 1, B40-8, Listeria monocytogenes, and Escherichia coli O157:H7 on shredded iceberg lettuce and in residual wash water. J. Food Prot. 2009, 72, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anfruns-Estrada, E.; Bottaro, M.; Pintó, R.M.; Guix, S.; Bosch, A. Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad. Foods 2019, 8, 637. https://doi.org/10.3390/foods8120637
Anfruns-Estrada E, Bottaro M, Pintó RM, Guix S, Bosch A. Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad. Foods. 2019; 8(12):637. https://doi.org/10.3390/foods8120637
Chicago/Turabian StyleAnfruns-Estrada, Eduard, Marilisa Bottaro, Rosa M. Pintó, Susana Guix, and Albert Bosch. 2019. "Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad" Foods 8, no. 12: 637. https://doi.org/10.3390/foods8120637
APA StyleAnfruns-Estrada, E., Bottaro, M., Pintó, R. M., Guix, S., & Bosch, A. (2019). Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad. Foods, 8(12), 637. https://doi.org/10.3390/foods8120637






