Effects of Pre-Processing Short-Term Hot-Water Treatments on Quality and Shelf Life of Fresh-Cut Apple Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Pre-Processing Short-Term Hot-Water Treatment
2.3. Fresh-Cut Preparation and Sampling
2.4. Color Measurement
2.5. Tissue Strength
2.6. Total Soluble Solids, Total Titratable Acidity and Vitamin C Contents
2.7. Microbial Analysis
2.8. Statistical Analysis
3. Results
3.1. Color Evaluation
3.2. Tissue Strength
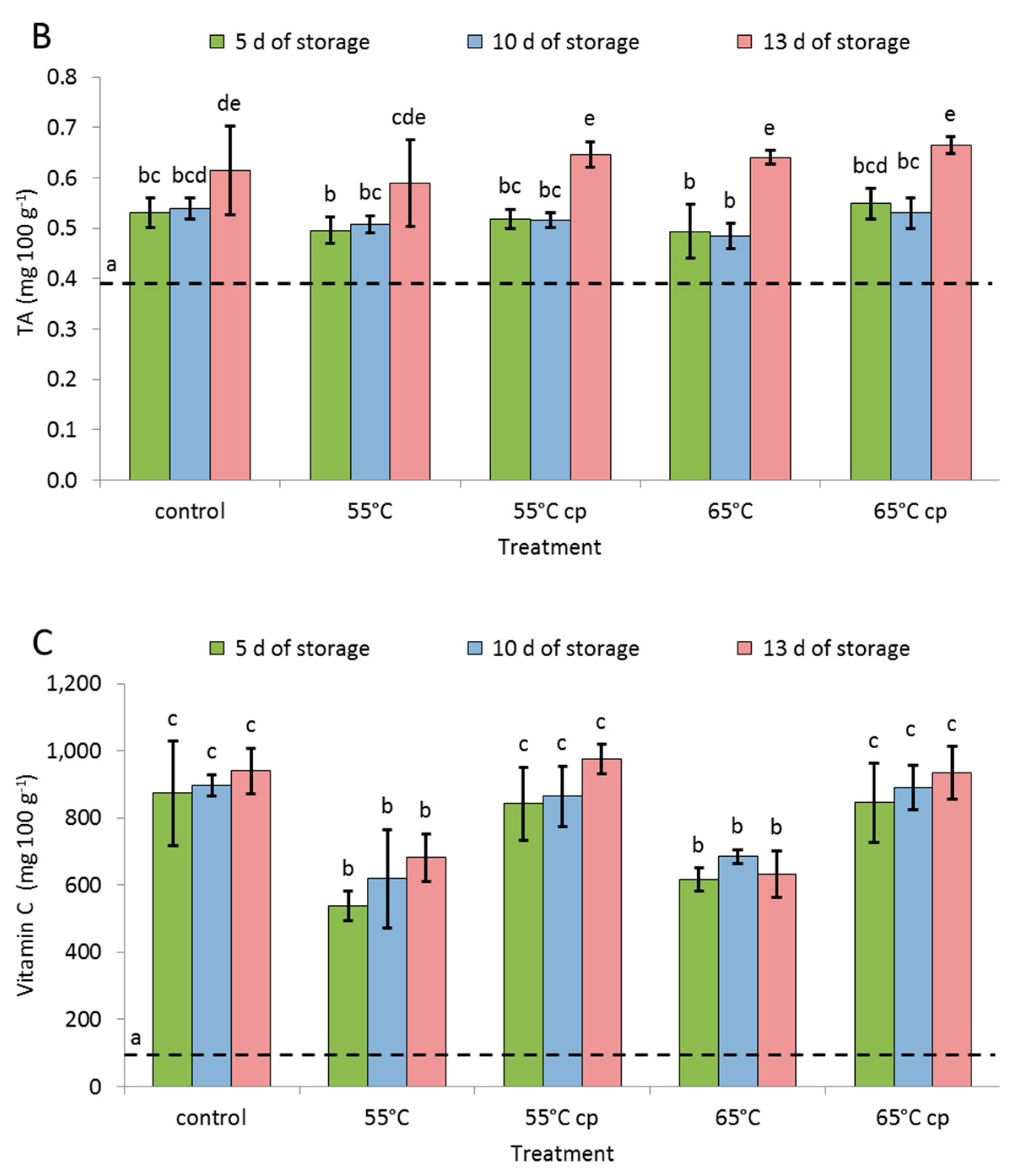
3.3. Total Soluble Solids, Total Titratable Acidity and Vitamin C
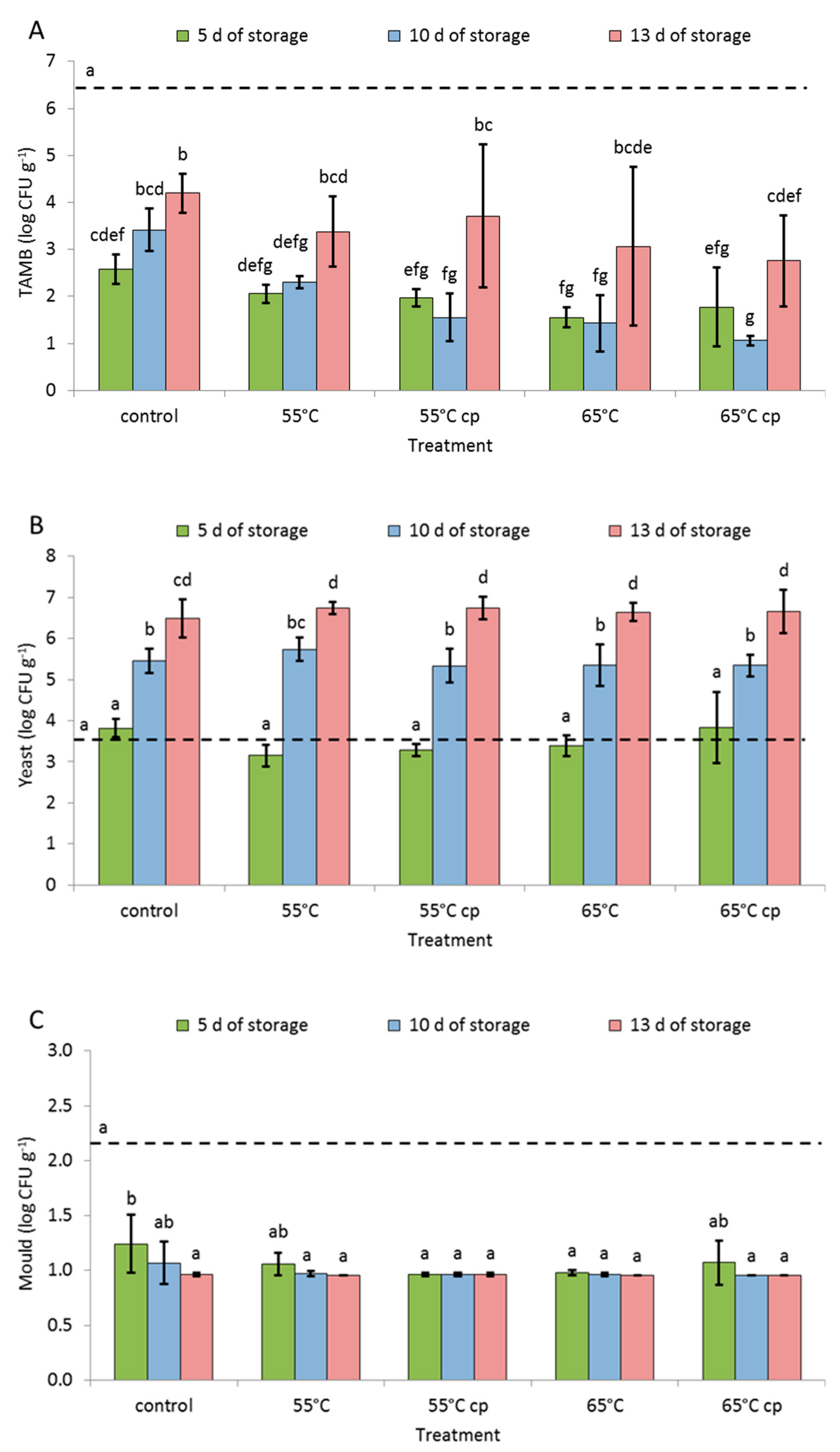
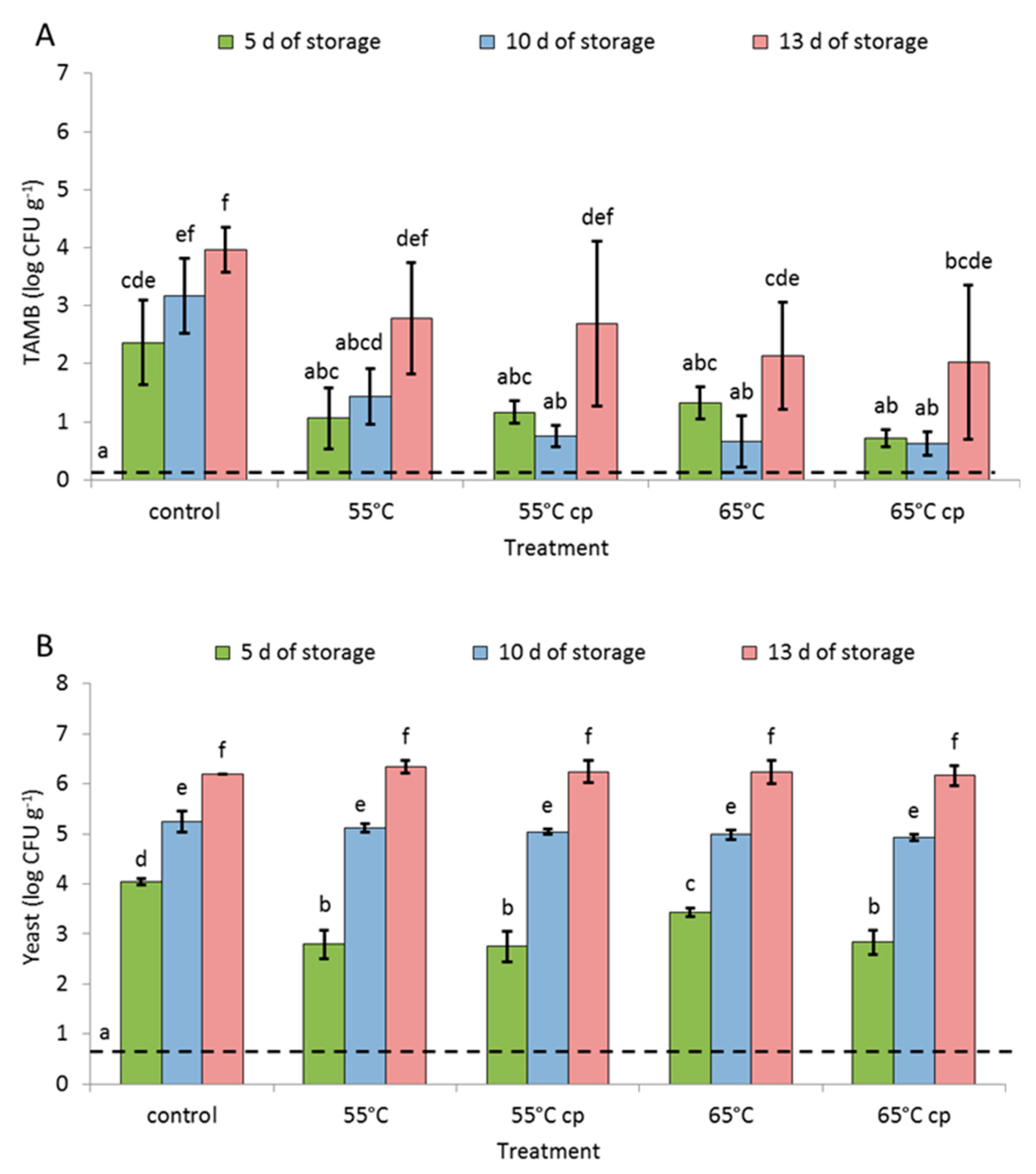
3.4. Microbial Analysis
4. Discussion
4.1. Tissue and Peel Color
4.2. Tissue Strength
4.3. Soluble Solid Content, Total Titratable Acidity and Vitamin C
4.4. Microbial Load
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toivonen, P.M.; DeEll, J.R. Physiology of Fresh-Cut Fruits and Vegetables. In Fresh-Cut Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2002; pp. 91–123. [Google Scholar]
- Beaudry, R.M. Response of horticultural commodities to low oxygen: Limits to the expanded use of MAP. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Watkins, C.B. Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. HortTechnology 2000, 10, 501–506. [Google Scholar] [CrossRef]
- Artés, F.; Gómez, P.A.; Artés-Hernández, F. Physical, physiological and microbial deterioration of minimally fresh processed fruits and vegetables. Rev. Agroquim. Tecnol. Aliment. 2007, 13, 177–188. [Google Scholar] [CrossRef]
- Capozzi, V.; Fiocco, D.; Amodio, M.L.; Gallone, A.; Spano, G. Bacterial stressors in minimally processed food. Int. J. Mol. Sci. 2009, 10, 3076–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rux, G.; Caleb, O.J.; Fröhling, A.; Herppich, W.B.; Mahajan, P.V. Respiration and storage quality of fresh-cut apple slices immersed in sugar syrup and orange juice. Food Bioprocess Technol. 2017, 10, 2081–2091. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.; Geyer, M.; Linke, M.; Pant, A.; Saengerlaub, S.; Caleb, O. Application of humidity-regulating tray for packaging of mushrooms. Postharvest Biol. Technol. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- Caleb, O.; Ilte, K.; Fröhling, A.; Geyer, M.; Mahajan, P. Integrated modified atmosphere and humidity package design for minimally processed Broccoli (Brassica oleracea L. var. italica). Postharvest Biol. Technol. 2006, 121, 87–100. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Grasa-Guillem, R.; Martín-Belloso, O. Quality changes in fresh-cut Fuji apple as affected by ripeness stage, antibrowning agents, and storage atmosphere. J. Food Sci. 2007, 72, S036–S043. [Google Scholar] [CrossRef]
- Koukounaras, A.; Diamantidis, G.; Sfakiotakis, E. The effect of heat treatment on quality retention of fresh-cut peach. Postharvest Biol. Technol. 2008, 48, 30–36. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- López-Rubira, V.; Conesa, A.; Allende, A.; Artés, F. Shelf life and overall quality of minimally processed pomegranate arils modified atmosphere packaged and treated with UV-C. Postharvest Biol. Technol. 2005, 37, 174–185. [Google Scholar] [CrossRef]
- Nishikawa, F.; Iwama, T.; Kato, M.; Hyodo, H.; Ikoma, Y.; Yano, M. Effect of sugars on ethylene synthesis and responsiveness in harvested broccoli florets. Postharvest Biol. Technol. 2005, 36, 157–165. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Tapia, M.S.; Argaíz, A.; Welli, J. Application of combined methods technology in minimally processed fruits. Food Res. Int. 1993, 26, 125–130. [Google Scholar] [CrossRef]
- Seide, K.; Thiem, I.; Viedt, H.; Wöhlke, A.; Guder, G. Vorzerkleinertes Obst und Gemüse Beurteilungsgrundlagen, BLL-Leitlinie Untersuchungsergebnisse Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit. 2017. Available online: https://www.laves.niedersachsen.de/download/118654/4._Vortrag_LAVES_Lebensmittel-_und_Veterinaerinstitut_Braunschweig_Hannover.pdf (accessed on 17 October 2019).
- Coupe, S.A.; Sinclair, B.K.; Watson, L.M.; Heyes, J.A.; Eason, J.R. Identification of dehydration-responsive cysteine proteases during post-harvest senescence of broccoli florets. J. Exp. Bot. 2003, 54, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrysiak, E.; Ganjyal, G.M. Apple peel morphology and attachment of Listeria innocua through aqueous environment as shown by scanning electron microscopy. Food Control 2018, 92, 362–369. [Google Scholar] [CrossRef]
- Kabelitz, T.; Hassenberg, K. Control of apple surface microflora for fresh-cut produce by post-harvest hot-water treatment. LWT Food Sci. Technol. 2018, 98, 492–499. [Google Scholar] [CrossRef]
- Lurie, S. Postharvest heat treatments. Postharvest Biol. Technol. 1998, 14, 257–269. [Google Scholar] [CrossRef]
- Trierweiler, B.; Schirmer, H.; Tauscher, B. Hot water treatment to control Gloeosporium disease on apples during long-term storage. J. Appl. Bot. 2003, 77, 156–159. [Google Scholar]
- Spadoni, A.; Guidarelli, M.; Phillips, J.; Mari, M.; Wisniewski, M. Transcriptional profiling of apple fruit in response to heat treatment: Involvement of a defense response during Penicillium expansum infection. Postharvest Biol. Technol. 2015, 101, 37–48. [Google Scholar] [CrossRef]
- Fallik, E. Prestorage hot water treatments (immersion, rinsing and brushing). Postharvest Biol. Technol. 2004, 32, 125–134. [Google Scholar] [CrossRef]
- Maxin, P.; Williams, M.; Weber, R.W. Control of fungal storage rots of apples by hot-water treatments: A Northern European perspective. Erwerbs-Obstbau 2014, 56, 25–34. [Google Scholar] [CrossRef]
- Roy, S.; Conway, W.S.; Watada, A.E.; Sams, C.E.; Erbe, E.F.; Wergin, W.P. Heat treatment affects epicuticular wax structure and postharvest calcium uptake in ‘Golden Delicious’ apples. HortScience 1994, 29, 1056–1058. [Google Scholar] [CrossRef] [Green Version]
- Lurie, S.; Fallik, E.; Klein, J.D. The effect of heat treatment on apple epicuticular wax and calcium uptake. Postharvest Biol. Technol. 1996, 8, 271–277. [Google Scholar] [CrossRef]
- Kabelitz, T.; Schmidt, B.; Herppich, W.B.; Hassenberg, K. Effects of hot water dipping on apple heat transfer and post-harvest fruit quality. LWT Food Sci. Technol. 2019, 108, 416–420. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Oms-Oliu, G.; Martín-Belloso, O. Effects of ripeness stages on the storage atmosphere, color, and textural properties of minimally processed apple slices. J. Food Sci. 2002, 67, 1958–1963. [Google Scholar] [CrossRef]
- Gunes, G.; Watkins, C.B.; Hotchkiss, J.H. Physiological responses of fresh-cut apple slices under high CO2 and low O2 partial pressures. Postharvest Biol. Technol. 2001, 22, 197–204. [Google Scholar] [CrossRef]
- Rocculi, P.; Romani, S.; Dalla Rosa, M. Evaluation of physico-chemical parameters of minimally processed apples packed in non-conventional modified atmosphere. Food Res. Int. 2004, 37, 329–335. [Google Scholar] [CrossRef]
- Cocci, E.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Changes in nutritional properties of minimally processed apples during storage. Postharvest Biol. Technol. 2006, 39, 265–271. [Google Scholar] [CrossRef]
- Cortellino, G.; Rizzolo, A.; Gobbi, S. Effect of conventional and alternative modified atmosphere packaging on the shelf-life of fresh-cut apples. Acta Hortic. 2013, 1071, 223–230. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Stauffer, C.E. Principles of Enzymology for the Food Sciences (2nd edn). Trends Food Sci. Technol. 1994, 5, 304. [Google Scholar]
- Chiabrando, V.; Giacalone, G. Effect of antibrowning agents on color and related enzymes in fresh-cut apples during cold storage. J. Food Proc. Preserv. 2012, 36, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.M.; Smith, N.L.; Lee, C.Y. Apple cultivar variations in response to heat treatment and minimal processing. J. Food Sci. 1993, 58, 1111–1114. [Google Scholar] [CrossRef]
- Kurenda, A.; Zdunek, A.; Schlüter, O.; Herppich, W.B. VIS/NIR spectroscopy, chlorophyll fluorescence, biospeckle and backscattering to evaluate changes in apples subjected to hydrostatic pressures. Postharvest Biol. Technol. 2014, 96, 88–98. [Google Scholar] [CrossRef]
- Herppich, W.B.; Herold, B.; Geyer, M.; Gomez, F. Effects of temperature and water relations on carrots and radish tuber texture. J. Appl. Bot. 2004, 78, 11–17. [Google Scholar]
- Herppich, W.B.; Huyskens-Keil, S.; Kadau, R. Effects of short-term low-temperature storage on mechanical and chemical properties of white Asparagus cell walls. J. Appl. Bot. Food Qual. 2005, 79, 63. [Google Scholar]
- Fan, L.; Song, J.; Forney, C.F.; Jordan, M.A. Fruit maturity affects the response of apples to heat stress. Postharvest Biol. Technol. 2011, 62, 35–42. [Google Scholar] [CrossRef]
- Ponting, J.D.; Jackson, R.; Watters, G. Refrigerated apple slices: Preservative effects of ascorbic acid, calcium and sulfites. J. Food Sci. 1972, 37, 434–436. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Z.; McHugh, T.H. Effect of dipping treatments on color stabilization and texture of apple cubes for infrared dry blanching process. J. Food Process Preserv. 2007, 31, 632–648. [Google Scholar] [CrossRef]
- Gorny, J.R.; Cifuentes, R.A.; Hess-Pierce, B.; Kader, A.A. Quality changes in fresh-cut pear slices as affected by cultivar, ripeness stage, fruit size, and storage regime. Int. J. Food Sci. Technol. 2000, 65, 541–544. [Google Scholar] [CrossRef]
- Biegańska-Marecik, R.; Czapski, J. The effect of selected compounds as inhibitors of enzymatic browning and softening of minimally processed apples. Acta Sci. Pol. Technol. Aliment. 2007, 6, 37–49. [Google Scholar]
- Maxin, P.; Weber, R.W.; Pedersen, H.L.; Williams, M. Hot-water dipping of apples to control Penicillium expansum, Neonectria galligena and Botrytis cinerea: Effects of temperature on spore germination and fruit rots. Eur. J. Hortic. Sci. 2012, 77, 1–9. [Google Scholar]
- Maxin, P.; Weber, R.W.; Pedersen, H.L.; Williams, M. Control of a wide range of storage rots in naturally infected apples by hot-water dipping and rinsing. Postharvest Biol. Technol. 2012, 70, 25–31. [Google Scholar] [CrossRef]
- Chand-Goyal, T.; Spotts, R.A. Enumeration of bacterial and yeast colonists of apple fruits and identification of epiphytic yeasts on pear fruits in the Pacific Northwest United States. Microbiol. Res. 1996, 151, 427–432. [Google Scholar] [CrossRef]
- DGHM. Microbiological Guiding and Warning Limits for Food Stuffs; German Society of Hygiene and Microbiology: Hannover, Germany, 2011. [Google Scholar]
- Tapia de Daza, M.S.; Alzamora, S.M.; Chanes, J.W.; Gould, G. Combination of preservation factors applied to minimal processing of foods. Crit. Rev. Food Sci. Nutr. 1996, 36, 629–659. [Google Scholar] [CrossRef]


| Treatment | sHWT | cp | Immersed in Sugar Syrup | Sampling Day |
|---|---|---|---|---|
| initial | no | no | no | 0 |
| control | no | yes | yes | 5, 10, 13 |
| 55 | 55 °C | no | ||
| 55_cp | 55 °C | yes | ||
| 65 | 65 °C | no | ||
| 65_cp | 65 °C | yes |
| day | Treatment | BI | L* | a* | b* |
|---|---|---|---|---|---|
| 0 | initial | 35.3 ± 6.0 (a) | 67.0 ± 1.7 (a) | 2.8 ± 1.1 (a) | 18.8 ± 2.5 (a) |
| 5 | control | 32.5 ± 1.0 (a) | 63.9 ± 0.4 (bc) | 2.3 ± 0.2 (ab) | 16.7 ± 0.2 (b) |
| 55 | 33.2 ± 1.8 (a) | 65.1 ± 1.1 (b) | 1.8 ± 0.0 (b) | 17.7 ± 0.9 (ab) | |
| 55_cp | 35.0 ± 1.5 (a) | 63.3 ± 0.8 (bc) | 2.2 ± 0.3 (ab) | 17.7 ± 0.5 (ab) | |
| 65 | 34.3 ± 3.5 (a) | 64.5 ± 0.7 (bc) | 2.1 ± 0.3 (ab) | 17.8 ± 1.4 (ab) | |
| 65_cp | 32.8 ± 0.9 (a) | 64.5 ± 0.7 (bc) | 2.1 ± 0.2 (ab) | 17.2 ± 0.3 (ab) | |
| 10 | control | 31.2 ± 0.6 (a) | 64.0 ± 0.1 (bc) | 2.2 ± 0.3 (ab) | 16.2 ± 0.2 (b) |
| 55 | 32.4 ± 1.6 (a) | 64.6 ± 1.1 (bc) | 2.2 ± 0.6 (ab) | 17.0 ± 0.1 (ab) | |
| 55_cp | 31.7 ± 1.3 (a) | 64.3 ± 1.2 (bc) | 2.1 ± 0.1 (ab) | 16.6 ± 0.3 (b) | |
| 65 | 34.1 ± 3.6 (a) | 64.7 ± 1.3 (bc) | 2.5 ± 0.5 (ab) | 17.6 ± 1.1 (ab) | |
| 65_cp | 33.7 ± 0.7 (a) | 62.8 ± 1.0 (c) | 2.4 ± 0.1 (ab) | 16.9 ± 0.2 (ab) | |
| 13 | control | 33.2 ± 0.9 (a) | 63.7 ± 0.5 (bc) | 2.3 ± 0.2 (ab) | 17.0 ± 0.6 (ab) |
| 55 | 33.6 ± 1.4 (a) | 65.2 ± 1.4 (b) | 2.3 ± 0.3 (ab) | 17.6 ± 0.2 (ab) | |
| 55_cp | 32.8 ± 1.8 (a) | 63.9 ± 1.4 (bc) | 2.2 ± 0.1 (ab) | 16.9 ± 1.1 (ab) | |
| 65 | 34.7 ± 1.9 (a) | 64.4 ± 0.6 (bc) | 2.2 ± 0.2 (ab) | 18.0 ± 1.1 (ab) | |
| 65_cp | 32.9 ± 1.6 (a) | 64.3 ± 1.2 (bc) | 2.2 ± 0.3 (ab) | 17.1 ± 1.2 (ab) |
| day | Treatment | BI | L* | a* | b* |
|---|---|---|---|---|---|
| 0 | initial | 60.7 ± 6.3 (abc) | 52.9 ± 2.5 (abc) | 17.1 ± 3.3 (a) | 16.6 ± 1.8 (a) |
| 5 | control | 63.0 ± 2.1 (abcd) | 53.0 ± 4.4 (abc) | 13.9 ± 2.5 (ab) | 19.1 ± 3.7 (ab) |
| 55 | 65.6 ± 1.7 (abcde) | 55.6 ± 2.5 (bc) | 12.4 ± 2.0 (bc) | 21.9 ± 2.8 (bc) | |
| 55_cp | 61.8 ± 1.9 (abcd) | 55.6 ± 2.1 (abc) | 10.3 ± 1.4 (bcd) | 21.5 ± 0.9 (bc) | |
| 65 | 69.8 ± 4.7 (de) | 57.3 ± 2.2 (c) | 9.8 ± 3.3 (bcd) | 25.3 ± 4.1 (cd) | |
| 65_cp | 65.5 ± 2.7 (abcde) | 55.3 ± 0.5 (bc) | 11.2 ± 0.7 (bcd) | 22.3 ± 1.1 (bc) | |
| 10 | control | 59.3 ± 6.3 (ab) | 55.0 ± 2.3 (ab) | 12.0 ± 3.1 (bc) | 19.5 ± 0.7 (ab) |
| 55 | 61.7 ± 3.7 (abcd) | 56.5 ± 1.5 (abc) | 10.2 ± 0.8 (bcd) | 21.9 ± 1.4 (bc) | |
| 55_cp | 57.4 ± 4.6 (a) | 58.6 ± 2.9 (a) | 7.4 ± 2.5 (d) | 22.7 ± 3.2 (bc) | |
| 65 | 68.1 ± 7.3 (de) | 57.7 ± 3.1 (c) | 8.5 ± 2.4 (cd) | 25.4 ± 0.6 (cd) | |
| 65_cp | 64.8 ± 5.4 (abcde) | 57.0 ± 2.0 (abc) | 8.2 ± 2.2 (cd) | 24.2 ± 2.8 (cd) | |
| 13 | control | 65.3 ± 3.4 (abcde) | 55.8 ± 2.6 (bc) | 12.2 ± 2.0 (bc) | 21.8 ± 1.5 (bc) |
| 55 | 66.6 ± 1.8 (bcde) | 58.0 ± 1.0 (c) | 8.8 ± 2.2 (cd) | 25.0 ± 1.8 (cd) | |
| 55_cp | 63.8 ± 1.7 (abcde) | 58.9 ± 1.2 (abc) | 6.8 ± 2.3 (d) | 25.4 ± 1.8 (cd) | |
| 65 | 72.1 ± 3.1 (e) | 57.9 ± 1.2 (c) | 7.4 ± 0.8 (d) | 27.4 ± 1.8 (d) | |
| 65_cp | 67.8 ± 2.1 (cde) | 57.3 ± 3.0 (c) | 9.3 ± 2.5 (cd) | 24.9 ± 3.2 (cd) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rux, G.; Efe, E.; Ulrichs, C.; Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Effects of Pre-Processing Short-Term Hot-Water Treatments on Quality and Shelf Life of Fresh-Cut Apple Slices. Foods 2019, 8, 653. https://doi.org/10.3390/foods8120653
Rux G, Efe E, Ulrichs C, Huyskens-Keil S, Hassenberg K, Herppich WB. Effects of Pre-Processing Short-Term Hot-Water Treatments on Quality and Shelf Life of Fresh-Cut Apple Slices. Foods. 2019; 8(12):653. https://doi.org/10.3390/foods8120653
Chicago/Turabian StyleRux, Guido, Efecan Efe, Christian Ulrichs, Susanne Huyskens-Keil, Karin Hassenberg, and Werner B. Herppich. 2019. "Effects of Pre-Processing Short-Term Hot-Water Treatments on Quality and Shelf Life of Fresh-Cut Apple Slices" Foods 8, no. 12: 653. https://doi.org/10.3390/foods8120653





