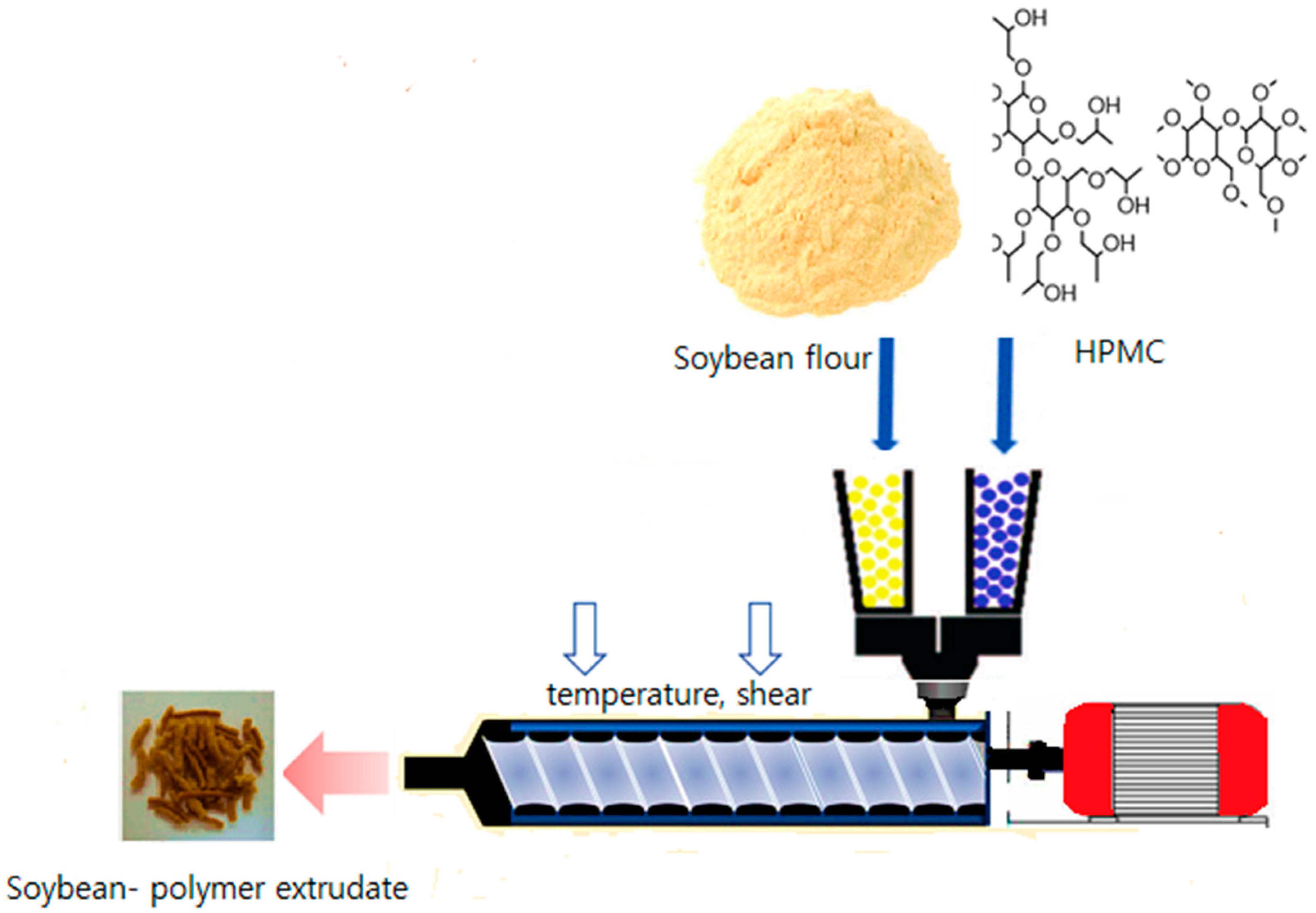
1. Introduction
Soybean (
Glycine max L.) is one of the healthiest legume food crops worldwide. It is a rich source of phenolic acids and flavonoids. The main flavonoids in soybean are isoflavones, a glycoside of genistin and daidzin that has a limited distribution in nature, except for soybeans [
1]. Isoflavones have a high level of antioxidant capacity [
2], which protects the body from various disorders, including cancer [
3], cardiovascular diseases [
4], and osteoporosis [
5].
The health benefits of the bioactive compounds primarily depend on to what extent they are released from the matrix, called “bioaccessibility”, which predicts the compounds available for intestinal absorption, called “bioavailability” [
6]. According to the Biopharmaceutics Classification System (BCS), isoflavones belong to class II are considered the most bioavailable but have poor bioaccessibility—for instance, the aqueous solubility and bioavailability of daidzin is 0.04 mg/mL and 40%, respectively [
7,
8]. Amidon et al. [
9] stated that particle size reduction, an increase in amorphization, and solid dispersion are important factors to improving the bioaccessibility of class II compounds.
Many approaches have been studied to improve the solubility of the poorly water-soluble compound. Reduction of the particle size is one of the most effective ways to increase the solubility [
10]. Hot melt extrusion is a novel processing technology in developing nano-size particles by the top-down technique (
Figure 1). In this technique, the coarse particle is to become nano sized by high shear forces of hot melt extrusion (HME) [
11].
The application of biopolymers is getting consideration in the food industry to improve food functionality. Hydroxypropyl methylcellulose (HPMC) is a safe food additive recommended by the European Food Safety Authority [
12]. HPMC is hydrophilic and biocompatible cellulose, mainly used in the food industry as a stabilizer, emulsifier, and protective colloid [
13]. It has the capabilities of hydration and gel formation, which enhance solubility, prolong the releasing time, and control the rheological characteristics of the active ingredients [
14,
15]. Crowley et al. [
16] stated that polymers facilitate the agglomeration of compounds into granules by steric hindrance and non-covalent bonding with the polymeric chain in aqueous media. To the best of our knowledge, HPMC polymers have not been used in soybean food products yet. Therefore, in this study, HPMC is added with soybean to develop food composites by HME, in order to improve the bioaccessibility and functionality of the phenolic compounds.
2. Materials and Methods
Soybean (Glycine max L.) flour was purchased from Chuncheon local market, Korea. Hydroxypropyl methylcellulose (HPMC) was purchased from Lotte food, Korea. Daidzin, glycitein, genistin, daidzein, glycitein, and genistein standards were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, United States). Phenolic reagent (Folin Ciocalteu, 2 N), sodium bicarbonate (Na2CO3), aluminum nitrate (AlNO3)3, potassium acetate (CH3COO2K), DPPH (2,2-diphenyl-1 picryl hydrazyl), phosphate buffer, trichloroacetic acid (TCA), ferric chloride, sulfuric acid, sodium phosphate, and ammonium molybdate were purchased from Merck Chemical Corp. (Darmstadt, Germany).
2.1. Preparation of Soybean-Polymer Composites
The purchased coarse soybean powder was pulverized by a low-temperature turbo mill (HKP-05; Korea Energy Technology Co., Ltd., Seoul, Korea). The temperature of the mill chamber was maintained at −18 °C. The ultrafine soybean powder was stored in a desiccator until further analysis.
Soybean food composite (SFC) was prepared by hot melt extrusion (STS-25HS twin-screw HME) (Hankook E.M. Ltd., Pyoung Taek, Korea), presented in
Table 1. The HME extruder was equipped with a round-shaped die (1 mm) at a feeding rate of 40 g/min at 150 rpm, with high shear of the twin screw. The processing temperature of HME was fixed at 80 °C and 130 °C. It has been reported that the processing parameter of the HME depends on the rheological characteristics of the polymer. The temperature of the HME must be higher than the glass transition temperature of a polymer to get good miscibility of the components [
17].
2.2. Particle Size Analysis
The SFC (soybean extrudate (SE), soybean (95%) plus HPMC (5%) (w/w) mixes (HSE)) and control powder (0.5 g) was suspended in 50 mL of distilled water. The supernatant was separated by centrifugation at 3000 rpm for 10 min. The particle size and the polydispersity index (PI) of the supernatant was studied using a light-scattering spectrophotometer (ELS-Z1000; Otsuka Electronics, Tokyo, Japan) with three replications.
2.3. Solubility Measurement
One gram of SFC and the control powder was suspended in 50 mL of distilled water at room temperature. The mixture was stirred for 1 h and then centrifuged at 5000 rpm for 10 min. The supernatant was decanted into an evaporating dish of known weight. Water absorption index (WAI), water solubility (WS), and swelling power (SP) were calculated by the following formulas, described by Piao et al. [
18].
2.4. Extraction Protocol for Phenolic Compound Analysis
The extraction protocol was followed according to the method described by Azad et al. [
19]. The SFC and control sample of 1 g was suspended in 100 mL of 80% ethanol and kept overnight in a shaker at room temperature. The extracts were filtered through Advantech 5B filter paper (Tokyo Roshi Kaisha Ltd., Saitama, Japan) and dried using a vacuum rotatory evaporator (EYLA N-1000, Tokyo, Japan) in a 40 °C water bath to obtain crude extracts. The crude extracts were freeze-dried to achieve a moisture content of <3%. Dried crude extracts were diluted using 80% ethanol to prepare 1 mg/mL stock solution and kept at −20 °C for further analysis.
2.5. Estimation of Total Phenolic and Flavonoid Content
Total phenolic (TP) content of SFC and control samples were determined by the Folin Ciocalteu assay [
20]. In brief, a sample aliquot of 1 mL of extract (1 mg/mL) was added to a test tube containing 0.2 mL of phenol reagent (1 N). The volume was increased by adding 1.8 mL of deionized water, and the solution was vortexed and left for 3 min for reaction. Furthermore, 0.4 mL of Na
2CO
3 (10% in water,
v/
v) was added, and the final volume (4 mL) was adjusted by adding 0.6 mL of deionized water. The absorbance was measured at 725 nm after incubation for 1 h at room temperature. The TP content was calculated from a calibration curve using gallic acid, and was expressed as mg/g of gallic acid equivalent (GAE).
The total flavonoid content (TF) content was quantified according to Ghimeray et al. [
21] with slight modifications. In short, a 0.5 mL aliquot of the sample (1 mg/mL) was mixed with 0.1 mL of 10% aluminum nitrate and 0.1 mL of potassium acetate (1 M) solution. To this mixture, 3.3 mL of distilled water was added to make a total volume of 4 mL. The mixture was vortexed and incubated for 40 mins. The TF content was measured using a spectrophotometer (UV-1800 240 V, Shimadzu Corporation, Kyoto, Japan) at 415 nm. The TF content was expressed as mg/g quercetin equivalents.
2.6. Analysis of a Single Isoflavone
The single isoflavone content was measured according to the modified method of Hsieh et al. [
22]. The sample extract (1 mg/mL) was filtered through a 0.45 µM syringe filter (Millipore, United States) before isoflavone analysis by high-performance liquid chromatography (HPLC). An HPLC system (CBM 20A, Shimadzu Co, Ltd., Kyoto, Japan) with two gradient pump systems (LC 20AT, Shimadzu), a C18 column (Kinetex, 100 × 4.6 mm, 2.6 micron, Phenomenex), an auto sample injector (SIL-20A, Shimadzu), a UV detector (SPD-10A, Shimadzu), and a column oven (35 °C, CTO-20A, Shimadzu) were used for analysis. Solvent A was 0.4% formic acid in water, and solvent B was acetonitrile. Gradient elution was used for 0–15 min, with 33–45% B; 15–30 min, with 45–55% B; 30–40 min, with 55–80% B; and 40–45 min, with 80–33% B. The flow rate was 1.0 mL/min, the injection volume was 10 μL, and the detection wavelength was 280 nm. A standard curve was calibrated using pure standards of soybean isoflavones with high linearity (
r2 > 0.995) by plotting the peak area of standard samples. The parameters like linearity, sensitivity, limit of detection (LOD), and limit of quantification (LOQ) of the HPLC method for isoflavones were validated according to the method of Montero et al. [
23]. All samples were analyzed in triplicate, and the single isoflavone content was expressed as mg/g.
2.7. Antioxidant Assays
2.7.1. DPPH Free Radical Scavenging Capacity
The antioxidant capacity was determined on the basis of the scavenging activity of the stable 2,2-diphenyl-1 picryl hydrazyl (DPPH) free radical, according to methods described by Braca et al. [
24]. The DPPH solution was prepared with 5.914 mg of DPPH powder in 100 mL of 100% methanol in the dark. Then, 1 mL of sample extract (1 mg/mL) was added to 3 mL of DPPH solution. The blank sample was prepared with 1 mL of distilled water instead of sample extract in 3 mL of DPPH solution. The mixture was shaken vigorously and left to stand at room temperature in the dark for 30 min. The absorbance was measured at 517 nm using a spectrophotometer (UV-1800 240 V, Shimadzu Corporation, Kyoto, Japan). The percent inhibition activities of the SFC and control were calculated against a blank sample using the following equation:
2.7.2. Ferric-Reducing Antioxidant Power Assay
The reducing power of the SFC and control samples was estimated according to the ferric reducing antioxidant power assay (FRAP) assay, as described by Pulido et al. [
25] with slight modification. In brief, 1 mL of extract (1 mg/mL) was mixed with 1 mL of 0.2 M phosphate buffer, maintaining a pH of 6.6. The mixture was then incubated at 50 °C for 20 min. After incubation, 1 mL of trichloroacetic acid (TCA) was added to the solution and centrifuged at 3000 rpm for 10 min. The collected supernatant was diluted with distilled water at a 1:1 ratio. Finally, 0.25 mL of 0.1% ferric chloride was added, and the absorbance was measured at 700 nm by a spectrophotometer.
2.7.3. Phosphomolybdenum Method
The total antioxidant capacity of SFC and control was assayed according to the phosphomolybdenum method (PPMD) method described by Prieto et al. [
26], with slight modification. In brief, 1 mL of extract solution (1 mg/mL) was added to 3 mL of 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate solution. The reaction mixture was incubated at 95 °C for 150 min. The absorbance of the mixture was measured at 695 nm by spectrophotometer against a blank sample. The antioxidant capacity was expressed as the absorbance of the sample.
3. Statistical Analysis
All data were expressed as mean ± standard deviation (SD) of triplicate measurements. The obtained results were compared among the soybean-polymer composite using a paired t-test, in order to observe the significant differences at the level of 5%. The paired t-test between the mean values of the SFC and control were analyzed by MINITAB (version 17.0, Minitab Inc., State College, PA, United States).
4. Results and Discussion
4.1. Analysis of Particle Size and Water Solubility
Particle size and distribution are important parameters of a solid formulation that estimates the rate of membrane permeability and availability of the compound in the intestine [
27]. It is clearly observed from
Table 2 that particle size is significantly reduced in SFC compared to the control. The nanoparticle size was recorded at 171.5 nm at 130 °C in HSE, which is significantly higher than the control (917 nm) (
Table 2).
Figure 2 shows the particle size distribution before and after extrusion, referred to as “polydispersity index” (PI). A splitting and wide range of particle distribution (0.52%) were observed in the non-extrudate sample, whereas narrowed particle size distribution was found in the extrudate sample (0.23%).
It is well-documented that HME is an efficient processing method to reduce particle size by a top-down technique. The high shear force generated by the twin screw of HME reduces the particle size from micro to nano [
11]. Reduction of particle size is associated with transitions from the crystalline state to an amorphous state by high-energy process [
28]. Enhancing bioaccessibility and bioavailability by the application of high shear is a commonly used process to obtain nanoparticles of drug compounds [
29]. A decrease in particle size leads to an increase in dissolution rate through an increase in the specific surface area [
30].
The PI is an important factor that affects the physical stability of nanosuspension [
31]. A PI value below 0.5 indicates that the formulation is homogeneous [
32]. All PI values of all samples, except the control, were below 0.4, thus showing that all samples formed homogeneous nanosuspensions.
The water absorption ability of the SFC and the control were measured by the parameter of the water solubility (WS), water absorption index (WAI), and swelling power (SP), as shown in
Table 3. It is noted that the highest WS (35.18%) was achieved in HSE at 130 °C, as compared to the control (7.14%). The SP was reduced in polymer-mediated formulation compared to the control; however, there was no significant difference observed for WAI among the formulations.
Aqueous solubility is an important limiting factor for the bioavailability of the BCS class II compounds. Water solubility and swelling power provide evidence of interactions between the water molecules and the starch chains in the crystalline and amorphous regions of a matrix. The water absorption index represents the ability of a substance to associate with water under a limited water condition.
Miller et al. [
33] investigated the impact of HPMC polymers on the ability to improve the solubility and bioavailability of the active drug compound process by HME. They stated that HPMC improves solubility due to its ability to provide hydrogen bonding donor sites. The formation of hydrogen bonding prevents migration of the active molecules within the polymer matrix. Zheng et al. [
34] explored solubility improvement of poorly water-soluble active molecules, by producing solid dispersions with HPMC processed by HME. The efficacy of the HPMC to enhance the solubility of the poorly soluble compound is largely due to its ability to prevent recrystallization of the molecules in aqueous media [
35,
36]. The swelling behavior of the extrudate soybean composite tends to be reduced, which may be due to the hydrolysis of starch in the extrudate soybean-polymer composite. Tester and Morrison [
37] stated that the swelling behavior of cereal is reduced due to amylose, which acts as an inhibitor of swelling. The WAI and SP were found to be reduced in corn, which is thought to be due to the collapse of the fibrous structures by a high shearing force of the twin screw [
38].
4.2. Analysis of Bioactive Compounds and Antioxidant Capacity
In this study, the highest extraction of total phenolic (9.82 mg/g) and flavonoid content (4.27 mg/g) was achieved in HSE at 130 °C, compared to the control (5.40 mg/g, 1.82 mg/g, respectively) (
Table 4). Glycosides and aglycones of soybean isoflavones, including daidzin, daidzein, glycitein, genistein, and genistin were extracted by 1.70, 0.82, 0.58, 1.02, and 1.84 mg/g, respectively, in HSE at 130 °C, which is significantly higher than the control (
Table 5). The total antioxidant capacity of the extrudate formulation was higher in HSE at 130 °C, measured by DPPH, FRAP, and PPMD (
Figure 3,
Figure 4 and
Figure 5).
The increase of the phenolic and flavonoid may be owing to the increase in the extractability of bound phenolics by the thermal degradation of the cellular constitutes [
14]. Youna et al. [
39] showed that reduced bran particle size by mechanical treatment resulted in increased bioaccessibility of phenolic acid. The increase of the phenolic profile in other food materials during extrusion processing has also been reported by another researcher [
40,
41]. Mahungu et al. [
42] observed that acetyl derivatives of genistin and daidzin are increased with increasing extrusion temperature. High temperature cause malonyl conjugates to undergo heat-induced decarboxylation and desertification of isoflavone derivatives.
The increased antioxidant property after processing might be because of the breakdown of cellular constituents and membranes of the materials. The formation of a variety of intermediate byproducts during processing might contribute to antioxidant properties [
43]. Denaturation of proteins during extrusion leads to open loose structures, which promote tannin-protein interaction and form tannin-protein complexes that enhance antioxidant capacity [
44]. White et al. [
45] observed that antioxidant activity is increased in extrudate products with elevated barrel temperature.
The bioaccessibility of the phenolic compound from soybean-polymer composites was increased in 130 °C. In general, the processing temperature in the HME extruder needs to be above the glass transition temperature of a given polymer to embed the active compound into a polymeric matrix [
45]. In this study, the increased bioaccessibility of phenolic compounds verified good miscibility between the soybean compound and HPMC polymer at 130 °C compared to 80 °C. Riaz et al. [
46] reported that temperature-sensitive phenolic compounds may undergo decarboxylation during extrusion, which reduces their bioaccessibility and antioxidant capacity. However, the addition of a polymer extends the thermal stability of active compounds in a form of the solid formulation. HPMC has proven to be an excipient for providing thermal stability to the low thermal stable compound during processing [
47].
5. Conclusions
HPMC polymers significantly increased the functional quality of melt extrudate soybean. It was observed that nano-size particles, higher solubility, and increased phenolic content and antioxidant capacity were achieved in a polymer-mediated soybean composite when processed at 130 °C. Therefore, it would be concluded that HPMC polymers could potentially be used in order to improve the bioaccessibility of bioactive compounds of soybean processed by HME.
Author Contributions
M.O.K.A. analyzed data and wrote the manuscript, W.W.K. performed the experiment, C.W.J., W.S.K. and C.H.P. revised the manuscript, and D.H.C. designed and supervised the study.
Funding
This study was supported by the Kangwon National University and the Institute of Bioscience and Biotechnology, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Messina, M.; Messina, V. Soyfoods, soybean isoflavones, and bone health: A brief overview. J. Ren. Nutri. 2000, 10, 63–68. [Google Scholar] [CrossRef]
- Malencic, D.; Popovic, M.; Miladinovic, J. Phenolic content and antioxidant properties of soybean (Glycine max L. Merr.) seeds. Molecules 2007, 12, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces G2/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Cell Biol. Toxi. 2008, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Crisafulli, A.; Marini, H.; Esposito, M.; D’Anna, R.; Corrado, F.; Bitto, A.; Squadrito, F. Cardiovascular effects of the phytoestrogen genistein. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2004, 2, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Pharmacological activities of Genistein, an isoflavone from soy (Glycine max L): Part II—Anti-cholesterol activity, effects on osteoporosis and menopausal symptoms. Int. J. Exp. Biol. 2001, 39, 520–525. [Google Scholar]
- Carbonell, C.J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frigola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Comp. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.H.; Yu, H.J.; Cho, N.S.; Kim, T.H.; Kim, D.C.; Chung, C.B.; Hwang, Y.I.; Kim, K.H. Enhanced bioavailability of soy isoflavones by complexation with β-cyclodextrin in rats. Biosci. Biotech. Biochem. 2004, 71, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Ame. J. Clinic. Nutri. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Amidon, G.E.; He, X.; Hageman, M.J. Physicochemical characterization and principles off oral doses form selection. In Burgers Medical Chemistry and Drug Discovery; Abraham, D.J., Ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Emeje, M.O.; Obidike, I.C.; Akpabio, E.I.; Ofoefule, S.I. Nanotechnology in drug delivery. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; In Tech: Rijeka, Croatia, 2012; p. 70. [Google Scholar]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Douroumis, D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev. Indus. Pharma. 2012, 392, 218–227. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipi, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Scientific Opinion on the safety of low-substituted hydroxypropyl cellulose (L-HPC) to be used as a food additive in food supplements in tablet form. EFSA 2018, 16, 5062. [Google Scholar]
- Garcıa, M.A.; Ferrero, C.; Bertola, N.; Martino, M.; Zaritzky, N. Edible coatings from cellulose derivatives to reduce oil uptake in fried products. Inno. Food Sci. Emer. Technol. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Delivery Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polymers. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thmma, S.; Upaddhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical application of hot melt extrusion: Part I. Drug Dev. Indus. Pharma. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- DiNunzio, J.; Brough, C.; Hughley, J.R.; Miller, D.A.; Williams, R.O., III; McGinity, J.W. Fusion production of solid dispersions containing a heat sensitive active ingredient by hot melt extrusion and kinetisol dispersing. Euro. J. Pharma. Biopharma. 2010, 74, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Lee, J.Y.; Weon, J.B.; Ma, C.J.; Ko, H.J.; Kim, D.D.; Kang, W.-S.; Cho, H.-J. Angelica gigas Nakai and soluplus-based solid formulations prepared by hot melting extrusion: Oral absorption enhancing and memory ameliorating effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.O.K.; Piao, J.P.; Park, C.H.; Cho, D.H. Far infrared irradiation enhances nutraceutical compounds and antioxidant properties in Angelica gigas Nakai powder. Antioxidants 2018, 7, 189. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphor-molybdic-phosphotungstic acid reagents. Ame. J. Enology Viticul. 1963, 16, 144–458. [Google Scholar]
- Ghimeray, A.K.; Sharma, P.; Hu, W.; Cheng, W.; Park, C.H.; Rho, H.S.; Cho, D.H. Far infrared assisted conversion of isoflavones and its effect on total phenolics and antioxidant activity in black soybean seed. J. Med. Plants Res. 2013, 7, 1129–1137. [Google Scholar]
- Hsieh, H.C.; Kao, T.H.; Chen, B.H. A Fast HPLC method for analysis of isoflavones in soybean. J. Liqu. Chroma. Related Techno. 2003, 27, 315–324. [Google Scholar] [CrossRef]
- Montero, G.; Gunther, G.; Valdés, K.; Arriagada, F.; Morales, J. An HPLC Method for the Determination of Isoflavones and the Evaluation of Their Antioxidant Capacity in Both Homogeneous and Microheterogeneous Systems. J. AOAC Intl. 2018, 101, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Fico, G.; Morelli, I.; Simone, F.; Tome, F.; Tommasi, N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharmacol. 2003, 8, 63–67. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura, C.F. Antioxidant activity of dietary polyphenols as determined by modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Sinko, P.J.; Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Science: Physical-Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 565. [Google Scholar]
- Muller, R.; Moschwitzer, J.; Bushrab, F. Manufacturing of nanoparticles by milling and homogenization techniques. In Nanoparticle Technology for Drug Delivery; Gupta, R., Kompella, U., Eds.; Taylor and Francis: New York, NY, USA, 2006; pp. 21–51. [Google Scholar]
- Dong, Y.; Feng, S. Poly (d,l-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int. J. Pharm. 2007, 342, 208–214. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Ame. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, X.; Zhao, X.; Xu, H. Oleanolic acid nanosuspensions: Preparation, in-vitro characterization and enhanced hepatoprotective effect. J. Pharma. 2005, 57, 259–264. [Google Scholar] [CrossRef]
- Jahagirdar, K.H.; Bhise, K. Investigation of formulation variables affecting the properties of lamotrigine nanosuspension prepared by using high pressure homogenizer using factorial design. DARU J. Pharma. Sci. 2014, 3, 732–739. [Google Scholar]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGinity, J.W.; Williams, R.O., III. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral pH transition. Drug Dev. Indus. Pharm. 2008, 34, 890–902. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, R.; Tang, X.; Zheng, L. Part I: Characterization of solid dispersions of nimodipine prepared by hot melt extrusion. Drug Dev. Indus. Pharma. 2007, 33, 791–802. [Google Scholar] [CrossRef]
- Yokoi, Y.; Yonemochi, E.; Terada, K. Effect of sugar ester and hydroxypropylmethylcellulose on the physicochemical stability of amorphous cefditoren pivoxil in aqueous suspension. Int. J. Pharma. 2005, 290, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Guyton, M.E.; Huand, T.; Bauer, J.M.; Stefanski, K.J.; Lu, Q. Enhanced oral bioavailability of a poorly water-soluble drug PNU-91325 by supersaturatable formulation. Drug Dev. Indus. Pharma. 2004, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Ning, L.; Villota, R.; Artz, W. Modification of corn fiber through chemical treatments combination with twin-screw extrusion. Cereal Chem. 1991, 68, 632–636. [Google Scholar]
- Youna, H.; Ulla, H.; Anna-Maija, L.; Pekka, L.; Tanja, N.; Vieno, P.; Minnamari, E.; Xavier, R. Potential of dry fractionation of wheat bran for the development of food ingredients, part II: Electrostatic separation of particles. J. Cereal Sci. 2011, 53, 9–18. [Google Scholar]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; Gonzalez-Laredo, R.F.; Pulido-Alonso, J. Effect of processing on the antioxidant properties of extracts from Mexican barley (Hordeum vulgare) cultivar. Food Chem. 2010, 119, 903–906. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I.; Fernandez, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Mahungu, S.M.; Diaz-Mercado, S.; Li, J.; Schwenk, M.; Singletary, K.; Faller, J. Stability of isoflavones during extraction processing of soy/corn mixture. J. Agric. Food Chem. 1999, 47, 279–284. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Inno. Food Sci. Emer. Tech. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J. Agric. Food Chem. 2010, 58, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Asif, M.; Ali, R. Stability of vitamins during extrusion. Criti. Rev. Food Sci. Nutri. 2009, 49, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Coppens, K.; Hall, M.; Larsen, P.; Mitchell, S.; Nguyen, P.; Read, M.; Shrestha, U.; Walia, P. Thermal and rheological evaluation of pharmaceutical excipients for hot melt extrusion. Presented at the AAPS Annual Meeting and Exposition, Baltimore, MD, USA, 7–11 November 2004. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).