Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice
Abstract
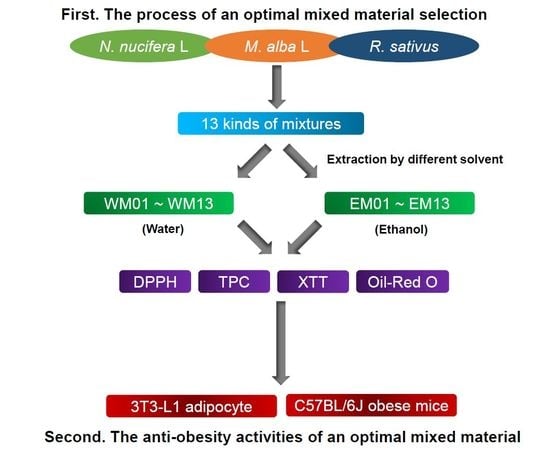
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Antioxidant Activity Analysis
2.3. Cell Culture and Differentiation
2.4. Cell Viability Assay
2.5. Oil-Red O Staining Assay
2.6. Animal Experiment Design
2.7. Glucose Tolerance Test
2.8. Serum Biochemical Parameter Analysis
2.9. Serum Adipokine Analysis
2.10. Real-Time Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
3.1. Effects of 26 Extracts by Mixture Ratio of N. Nucifera L., M. alba L., R. Sativus on the Antioxidant and Anti-Adipogenic Activities
3.2. Effect of EM01 on Lipid Accumulation
3.3. Effects of EM01 on Body Weight, Food Intake, FER, Organ Weight, and Adipose Tissue Weight in HFD-Induced Obese Mice
3.4. Effects of EM01 on glucose tolerance in HFD-induced obese mice
3.5. Effects of EM01 on the Serum Lipid Profile in HFD-Induced Obese Mice
3.6. Effects of EM01 on the Energy Balancing Metabolism in HFD-Induced Obese Mice
3.7. Effects of EM01 mRNA Expression Level of Lipid Metabolism-Related Genes in HFD-Induced Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moon, J.; Do, H.J.; Kim, O.Y.; Shin, M.J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. 2013, 58, 347–354. [Google Scholar] [CrossRef]
- Mu, M.; Xu, L.-F.; Hu, D.; Wu, J.; Bai, M.-J. Dietary patterns and overweight/obesity: A review article. Iran J. Public Health. 2017, 46, 869–876. [Google Scholar] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Correia, M.L.; Haynes, W.G.; Mark, A.L. Obesity-associated hypertension: New insights into mechanisms. Hypertension. 2005, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.; Claussen, B.; Hussain, A. High prevalence of obesity, dyslipidemia and metabolic syndrome in a rural area in Pakistan. Diabetes Metab. Syndr: Clinical Res. Rev. 2008, 2, 13–19. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. The Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef]
- Weigle, D.S. Pharmacological therapy of obesity: Past, present, and future. J. Clin. Endocrinol. Metab. 2003, 88, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46–59. [Google Scholar] [CrossRef]
- Chaturvedi, P. Inhibitory response of Raphanus sativus on lipid peroxidation in albino rats. J. Evid. Based Complementary Altern Med. 2008, 5, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Oh, J.; Kim, H.A.; Kim, Y.J.; Jeong, K.S.; Rhyu, D.Y. Anti-obesity effects of the mixture of Eriobotrya japonica and Nelumbo nucifera in adipocytes and high-fat diet-induced obese mice. Am. J. Chin. Med. 2015, 43, 681–694. [Google Scholar] [CrossRef]
- Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [Google Scholar]
- Nadeem, S. Synergistic effect of Commiphora mukul (gum resin) and Lagenaria siceraria (fruit) extracts in high fat diet induced obese rats. Asian Pac. J. Trop. Dis. 2012, S883–S886. [Google Scholar] [CrossRef]
- Jang, G.W.; Park, E.Y.; Choi, S.H.; Choi, S.I.; Cho, B.Y.; Sim, W.S.; Han, X.; Cho, H.D.; Lee, O.H. Development and validation of analytical method for wogonin, quercetin, and quercetin-3-O-glucuronide in extracts of Nelumbo nucifera, Morus alba L., and Raphanus sativus mixture. J. Food Hyg. Saf. 2018, 33, 289–295. [Google Scholar] [CrossRef]
- Cho, M.; Ko, S.B.; Kim, J.M.; Lee, O.H.; Lee, D.W.; Kim, J.Y. Influence of extraction conditions on antioxidant activities and catechin content from bark of Ulmus pumila L. Appl. Biol. Chem. 2016, 59, 329–336. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Cho, B.Y.; Park, M.R.; Lee, J.H.; Ra, M.J.; Han, K.C.; Kang, I.J.; Lee, O.H. Standardized Cirsium setidens Nakai ethanolic extract suppresses adipogenesis and regulates lipid metabolisms in 3T3-L1 adipocytes and C57BL/6J mice fed high-fat diets. J. Med. Food. 2017, 20, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, B.Y.; Choi, S.H.; Jung, T.D.; Choi, S.I.; Lim, J.H.; Lee, O.H. Sulforaphane attenuates bisphenol A-induced 3T3-L1 adipocyte differentiation through cell cycle arrest. J. Funct. Foods. 2018, 44, 17–23. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Gotto, A.M.; LaRosa, J.C.; Maroni, J.; Szarek, M.; Grundy, S.M.; Kastelein, J.P.; Bittner, V.; Fruchart, J.C. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 2007, 357, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shen, L.; Liu, K.J.; Tso, P.; Xiong, Y.; Wang, G.; Woods, S.C.; Liu, M. Anti-obesity and anti-hyperglycemic effects of ginsenoside Rb1 in rats. Diabetes 2010, 59, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Pardina, E.; Ferrer, R.; Baena-Fustegueras, J.A.; Lecube, A.; Fort, J.M.; Vargas, V.; Catalan, R.; Peinado-Onsurbe, J. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes. Surg. 2010, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [Google Scholar] [CrossRef]
- Pauwels, E.; Stoven, V.; Yamanishi, Y. Predicting drug side-effect profiles: A chemical fragment-based approach. BMC bioinformatics. 2011, 12, 169–182. [Google Scholar] [CrossRef]
- Choe, J.H.; Jang, A.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Lee, M.A.; Kim, H.W.; Kim, C.J. Antioxidant activities of lotus leaves (Nelumbo nucifera) and barley leaves (Hordeum vulgare) extracts. Food Sci. Biotechnol. 2010, 19, 831–836. [Google Scholar] [CrossRef]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Bin, X.; Jin, W.; Wenqing, W.; Chunyang, S.; Xiaolong, H.; Jianguo, F. Nelumbo nucifera alkaloid inhibits 3T3-L1 preadipocyte differentiation and improves high-fat diet-induced obesity and body fat accumulation in rats. J. Med. Plant Res. 2011, 5, 2021–2028. [Google Scholar]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLOS One. 2012, 7, e50619. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, F.; Canistro, D.; Sapone, A.; De Nicola, G.R.; Marquillas, C.B.; Iori, R.; Antonazzo, I.C.; Gentilini, F.; Paolini, M. Raphanus sativus cv. sango sprout juice decreases diet-induced obesity in Sprague Dawley rats and ameliorates related disorders. PLoS ONE 2016, 11, e0150913. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Cai, B.; Wang, K.; Wang, S.; Zhou, S.; Yu, X.; Xu, B.; Chen, L. Neferine enhances insulin sensitivity in insulin resistant rats. J. Ethnopharmacol. 2009, 124, 98–102. [Google Scholar] [CrossRef]
- Zhou, T.; Luo, D.; Li, X.; Luo, Y. Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J. Med. Plant. Res. 2009, 3, 290–293. [Google Scholar]
- Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Komosińska-Vassev, K.; Kucharz, E.; Kotulska, A.; Olczyk, K. Relationship between adiponectin, leptin, IGF-1 and total lipid peroxides plasma concentrations in patients with systemic sclerosis: Possible role in disease development. Int. J. Rheum Dis. 2016, 19, 706–714. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, W.-S.; Choi, S.-I.; Cho, B.-Y.; Choi, S.-H.; Han, X.; Cho, H.-D.; Kim, S.-H.; Lee, B.-Y.; Kang, I.-J.; Cho, J.-H.; et al. Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice. Foods 2019, 8, 170. https://doi.org/10.3390/foods8050170
Sim W-S, Choi S-I, Cho B-Y, Choi S-H, Han X, Cho H-D, Kim S-H, Lee B-Y, Kang I-J, Cho J-H, et al. Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice. Foods. 2019; 8(5):170. https://doi.org/10.3390/foods8050170
Chicago/Turabian StyleSim, Wan-Sup, Sun-Il Choi, Bong-Yeon Cho, Seung-Hyun Choi, Xionggao Han, Hyun-Duk Cho, Seung-Hyung Kim, Boo-Yong Lee, Il-Jun Kang, Ju-Hyun Cho, and et al. 2019. "Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice" Foods 8, no. 5: 170. https://doi.org/10.3390/foods8050170
APA StyleSim, W.-S., Choi, S.-I., Cho, B.-Y., Choi, S.-H., Han, X., Cho, H.-D., Kim, S.-H., Lee, B.-Y., Kang, I.-J., Cho, J.-H., & Lee, O.-H. (2019). Anti-Obesity Effect of Extract from Nelumbo Nucifera L., Morus Alba L., and Raphanus Sativus Mixture in 3T3-L1 Adipocytes and C57BL/6J Obese Mice. Foods, 8(5), 170. https://doi.org/10.3390/foods8050170