Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Preparation of NADESs
2.3. Preparation of ICS Leaves Powder
2.4. SHS-GC-MS Analysis Conditions
2.5. Preparation of ICS Samples for SHS-GC-MS Analysis
2.6. Determination of Retention Index
3. Results and Discussions
3.1. Optimization of SHS Parameters
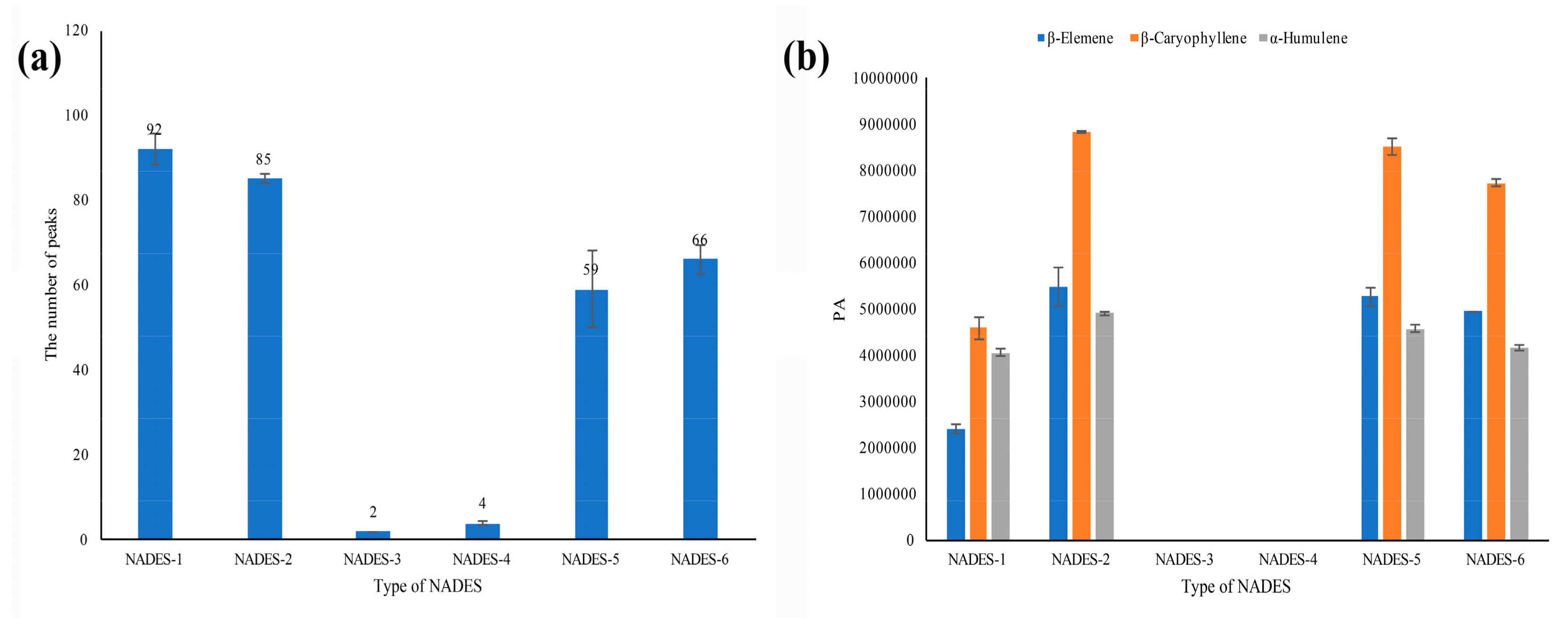
3.2. Selection of NADESs for SHS-GC-MS Analysis
3.3. Effects of the Solid–Liquid Ratio and Water Content of NADESs on SHS Efficiency
3.4. Volatile Components’ Characterization by SHS-GC-MS
3.4.1. Identification of Terpenoids
3.4.2. Identification of Aromatics
3.4.3. Identification of Aliphatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant Volatiles: Production, Function and Pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocolloid. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef]
- Da Silva, P.D.M.; De Lima, L.S.; Caetano, I.K.; Torres, Y.R. Comparative analysis of the volatile composition of honeys from Brazilian stingless bees by static headspace GC-MS. Food Res. Int. 2017, 102, 536–543. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 6. [Google Scholar] [CrossRef]
- Saeidi, K.; Moosavi, M.; Lorigooini, Z.; Maggi, F. Chemical characterization of the essential oil compositions and antioxidant activity from Iranian populations of Achillea wilhelmsii K. Koch. Ind. Crop. Prod. 2018, 112, 274–280. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Li, F.; Peng, S.; Chen, B. Different responses of invasive and native species to elevated CO2 concentration. Acta Oecol. 2009, 35, 128–135. [Google Scholar] [CrossRef]
- Lin, R.J.; Chen, C.Y.; Lo, W.L. Cytotoxic activity of Ipomoea cairica. Nat. Prod. Res. 2008, 22, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.Q.; Wang, J.S.; Luo, J.G.; Kong, L.Y. Novel acylated lipo-oligosaccharides from the tubers of Ipomoea batatas. Carbohyd. Res. 2009, 344, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.G.; Rao, S.; Lal, S. Mosquito larvicidal properties of essential oil of an indigenous plant, Ipomoea cairica Linn. Jpn. J. Infect. Dis. 2004, 57, 176–177. [Google Scholar] [PubMed]
- Meira, M.; Da Silva, E.P.; David, J.M.; David, J.P. Review of the genus Ipomoea: chemistry and biological activities. Rev. Bras. Farmacogn. 2012, 22, 682–713. [Google Scholar] [CrossRef]
- Li, J.H.; Pan, J.T.; Yin, Y.Q. Two novel resin glycosides isolated from Ipomoea cairica with α-glucosides inhibitory activity. Chin. J. Nat. Med. 2016, 14, 227–231. [Google Scholar] [PubMed]
- Yu, B.; Luo, J.; Wang, J.; Zhang, D.; Yu, S.; Kong, L. Pentasaccharide resin glycosides from Ipomoea cairica and their cytotoxic activities. Phytochemistry 2013, 95, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, L.; Zhang, W. Peroxidase from Ipomoea cairica (L) SW. Isolation, purification and some properties. Process Biochem. 1996, 31, 443–448. [Google Scholar]
- Ishak, A.R.; Dom, N.C.; Hussain, H.; Sabri, N.H. Biolarvacidal potential of Ipomoea Cairica extracts against key Dengue vevtors. Procedia Soc. Behav. Sci. 2014, 153, 180–188. [Google Scholar] [CrossRef]
- Samuel, L.; Lalrotluanga, R.; Muthukumaran, R.B.; Gurusubramanian, G.; Senthilkumar, N. Larvicidal activity of Ipomoea cairica (L) Sweet and Ageratina adenophora (Spreng.) King & H. Rob. plant extracts against arboviral and filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Exp. Parasitol. 2014, 141, 112–121. [Google Scholar] [PubMed]
- Ferreira, A.A.; Amaral, F.A.; Duarte, I.D.G.; Oliveira, P.M.; Alves, R.B.; Silveira, D.; Azevedo, A.O.; Raslan, D.S.; Castro, M.S.A. Antinociceptive effect from Ipomoea cairica extract. J. Ethnopharmacol. 2006, 105, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Tulukcu, E.; Cebi, N.; Sagdic, O. Chemical Fingerprinting of Seeds of Some Salvia Species in Turkey by Using GC-MS and FTIR. Foods 2019, 8, 118. [Google Scholar] [CrossRef]
- Nagyová, S.; Tölgyessy, P. Validation Including Uncertainty Estimation of a GC–MS/MS Method for Determination of Selected Halogenated Priority Substances in Fish Using Rapid and Efficient Lipid Removing Sample Preparation. Foods 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Sitaramaraju, Y.; Van Hul, A.; Wolfs, K.; Van Schepdael, A.; Hoogmartens, J.; Adams, E. Static headspace gas chromatography of (semi-) volatile drugs in pharmaceuticals for topical use. J. Pharmaceut. Biomed. 2008, 47, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Rustum, A. Development and validation of a fast static headspace GC method for determination of residual solvents in permethrin. J. Pharmaceut. Biomed. 2016, 128, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Varona-Torres, E.; Carlton, D.D.; Hildenbrand, Z.L.; Schug, K.A. Matrix-effect-free determination of BTEX in variable soil compositions using room temperature ionic liquid co-solvents in static headspace gas chromatography mass spectrometry. Anal. Chim. Acta. 2018, 1021, 41–50. [Google Scholar] [CrossRef]
- D’Autry, W.; Zheng, C.; Bugalama, J.; Wolfs, K.; Hoogmartens, J.; Adams, E.; Wang, B.; Van Schepdael, A. Liquid paraffin as new dilution medium for the analysis of high boiling point residual solvents with static headspace-gas chromatography. J. Pharmaceut. Biomed. 2011, 55, 1017–1023. [Google Scholar] [CrossRef]
- Wang, M.; Fang, S.; Liang, X.R. Natural deep eutectic solvents as eco-friendly and sustainable dilution medium for the determination of residual organic solvents in pharmaceuticals with static headspace-gas chromatography. J. Pharmaceut. Biomed. Anal. 2018, 158, 262–268. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta. 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Re. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Pereira, F.P.; Namiesnik, J. Ionic liquids and deep eutectic mixtures: sustainable solvents for extraction processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Kim, E.M.; Lee, J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solvents. Food Chem. 2018, 251, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fraige, K.; Arrua, R.D.; Sutton, A.T.; Funari, C.S.; Cavalheiro, A.J.; Hilder, E.F.; Da Silva Bolzani, V. Using natural deep eutectic solvents for the extraction of metabolites in Byrsonima intermedia leaves. J. Sep. Sci. 2019, 42, 591–597. [Google Scholar] [CrossRef]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018, 263, 193–199. [Google Scholar] [CrossRef]
- Ni, M.; Sun, T.; Zhang, L.; Liu, Y.; Xu, M.; Jiang, Y. Relationship study of partition coefficients between ionic liquid and headspace for organic solvents by HS-GC. J. Chromatogr. B 2014, 945, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zuo, X.B.; Xu, X.J.; Ren, D.H. Density, viscosity and excess molar volume of binary mixtures of tri-n-octylamine + diluents (n-heptane, n-octane, n-nonane, and n-decane) at various temperatures. J. Chem. Thermodyn. 2014, 68, 281–287. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their application. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Kim, S.; Koo, I.; Wei, X.L.; Zhang, X. A method of finding optimal weight factors for compound identification in gas chromatography-mass spectrometry. Bioinformatics 2012, 28, 1158–1163. [Google Scholar] [CrossRef]
- Koo, I.; Kim, S.; Zhang, X. Comparative analysis of mass spectral matching-based compound identification in gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1298, 132–138. [Google Scholar] [CrossRef]
- Lee, H.; Finckbeiner, S.; Yu, J.S.; Wiemer, D.F.; Eisner, T.; Attygalle, A.B. Characterization of (E, E)-farnesol and its fatty acid esters from anal scent glands of nutria (Myocasgor coypus) by gas chromatography-mass spectrometry and gas chromatography-infrared spectrometry. J. Chromatogr. A 2007, 1165, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kooa, I.; Shia, X.; Kimb, S.; Zhanga, X. iMatch2: Compound identification using retention index for analysis of gas chromatography-mass spectrometry data. J. Chromatogr. A 2014, 1337, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, M.; Li, N.; Li, Z.; Li, H.; Shao, S.; Zou, K.; Zou, L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem. Bioph. Res. Commun. 2017, 490, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, F.; Davis, R.W. Engineering Escherichia coli for the production of terpene mixture enriched in caryophyllene and caryophyllene alcohol as potential aviation fuel compounds. Metab. Eng. Commun. 2018, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ngamprasertsith, S.; Menwa, J.; Sawangkeaw, R. Caryophyllene oxide extraction from lemon basil (Ocimum citriodorum Vis.) straw by hydrodistillation and supercritical CO2. J. Supercrit. Fluid. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef] [PubMed]


| No. | Components | RT | Formula | CAS | Score | RI | Contents * (%) |
|---|---|---|---|---|---|---|---|
| 1 | 5-Methyl-5-hexen-2-ol | 2.07 | C7H14O | 50551-88-7 | 70.73 | 692 | 3.65 |
| 2 | 2-Ethyl-furan | 2.14 | C6H8O | 3208-16-0 | 77.37 | 708 | 1.41 |
| 3 | 1-(Methylencyclopropyl)-ethanol | 2.36 | C6H10O | 70.89 | 725 | 0.14 | |
| 4 | 4-Penten-1-ol | 2.69 | C5H10O | 821-09-0 | 65.28 | 766 | 0.43 |
| 5 | 2, 4-Dimethyl-3-pentanol | 3.10 | C7H16O | 600-36-2 | 80.18 | 803 | 3.75 |
| 6 | 3-Furaldehyde | 3.72 | C5H4O2 | 498-60-2 | 90.06 | 829 | 4.73 |
| 7 | (E)-2-hexenal | 4.15 | C6H10O | 6728-26-3 | 80.70 | 849 | 1.07 |
| 8 | 4-Methyl-2-penten-1-ol | 4.46 | C6H12O | 5362-55-0 | 67.13 | 863 | 0.59 |
| 9 | Heptanal | 5.42 | C7H14O | 111-71-7 | 67.55 | 904 | 0.27 |
| 10 | (+/-)-2-Amino-1-propanol | 5.61 | C3H9NO | 2749-11-3 | 72.19 | 910 | 0.13 |
| 11 | 1-(2-Furanyl)-ethanone, | 5.83 | C6H6O2 | 1192-62-7 | 60.92 | 916 | 0.30 |
| 12 | Butyrolactone | 6.26 | C4H6O2 | 96-48-0 | 63.77 | 923 | 0.21 |
| 13 | (1R)-2, 6, 6-Trimethylbicyclo [3.1.1] hept-2-ene | 6.50 | C10H16 | 7785-70-8 | 54.28 | 936 | 0.14 |
| 14 | 1-Methyl-3-cyclohexen-1-ol | 7.27 | C7H12O | 33061-16-4 | 53.39 | 959 | 0.12 |
| 15 | Benzaldehyde | 7.36 | C7H8O | 100-52-7 | 87.31 | 962 | 1.52 |
| 16 | 1-Methyl-pyrazole-4-carboxaldehyde | 7.53 | C5H6N2O | 25016-11-9 | 73.89 | 966 | 0.41 |
| 17 | 2-Methyl-1-hepten-6-one | 8.34 | C8H14O | 10408-15-8 | 69.68 | 991 | 0.19 |
| 18 | 2-Pentyl-furan | 8.46 | C9H14O | 3777-69-3 | 77.14 | 994 | 0.57 |
| 19 | 1-Methyl-1H-pyrrole-2-carboxaldehyde | 8.90 | C6H7NO | 1192-58-1 | 67.63 | 1004 | 0.31 |
| 20 | Benzeneacetaldehyde | 10.22 | C8H8O | 122-78-1 | 85.65 | 1023 | 0.81 |
| 21 | 2, 5-Furandicarboxaldehyde | 11.44 | C6H4O3 | 823-82-5 | 61.79 | 1042 | 0.19 |
| 22 | Nonanal | 12.27 | C9H18O | 124-19-6 | 74.41 | 1107 | 0.20 |
| 23 | Levomenthol | 12.38 | C10H20O | 2216-51-5 | 65.21 | 1110 | 0.16 |
| 24 | Phenylethyl alcohol | 12.57 | C8H10O | 60-12-8 | 73.38 | 1116 | 0.17 |
| 25 | 2, 6, 6-Trimethyl-1, 3-cyclohexadiene-1-carboxaldehyde | 15.27 | C10H14O | 116-26-7 | 61.78 | 1202 | 0.10 |
| 26 | 2, 6, 6-Trimethyl-1-cyclohexene-1-carboxaldehyde | 15.90 | C10H16O | 432-25-7 | 64.44 | 1204 | 0.16 |
| 27 | 4-(2, 6, 6-Trimethyl-1, 3-cyclohexadien-1-yl)-2-butanone | 17.11 | C13H20O | 20483-36-7 | 66.03 | 1266 | 0.20 |
| 28 | (1R, 2R, 5R, E)-7-Ethylidene-1, 2, 8, 8-tetramethylbicyclo [3.3.1] octane | 17.61 | C14H14 | 193695-14-6 | 54.09 | 1283 | 0.17 |
| 29 | Tetrahydro-6-propyl-2H-pyran-2-one | 17.79 | C8H14O2 | 542-28-9 | 69.71 | 1289 | 0.19 |
| 30 | 2, 6, 10, 10-Tetramethyl-1-oxaspiro [4.5] dec-6-ene | 18.17 | C13H22O | 36431-72-8 | 80.79 | 1302 | 0.25 |
| 31 | (3R-trans)-4-Ethenyl-4-methyl-3-(1-methylethenyl)-1-(1-methylethyl)-cyclohexene | 19.24 | C15H24 | 20307-84-0 | 73.27 | 1342 | 0.14 |
| 32 | trans-Calamenene | 19.57 | C15H22 | 73209-42-4 | 74.69 | 1355 | 0.14 |
| 33 | α-Cubebene | 20.28 | C15H24 | 17699-14-8 | 81.24 | 1381 | 0.17 |
| 34 | β-Elemene | 20.52 | C15H24 | 515-13-9 | 97.06 | 1389 | 0.63 |
| 35 | 2, 4, 6-Trimethyl-decane | 20.85 | C12H26 | 2801-84-5 | 51.23 | 1402 | 0.11 |
| 36 | δ-Selinene | 20.92 | C15H24 | 28624-23-9 | 71.98 | 1405 | 0.11 |
| 37 | 4-(Dimethylamine)-benzaldehyde | 21.02 | C9H11NO | 100-10-7 | 90.17 | 1409 | 0.90 |
| 38 | (E)-1-(2, 3, 6-trimethylphenyl) buta-1, 3-diene (TPB,1) | 21.29 | C13H16 | 1000357-25-7 | 58.90 | 1419 | 0.23 |
| 39 | β-Caryophyllene | 21.44 | C15H24 | 87-44-5 | 98.21 | 1424 | 22.68 |
| 40 | (+)-epi-Bicyclosesquiphellandrene | 21.67 | C15H24 | 54274-73-6 | 93.89 | 1435 | 0.75 |
| 41 | [1aR-(1aα, 7aα, 7bα)]-1a, 2, 3, 5, 6, 7, 7a, 7b-Octahydro-1, 1, 7, 7a-tetramethyl-1H-cyclopropa [a] naphthalene | 21.76 | C15H24 | 17334-55-3 | 82.99 | 1439 | 0.32 |
| 42 | (1S-cis)-1, 2, 3, 5, 6, 8a-Hexaahydro-4, 7-dimethyl-1-(1-methylethyl)-naphthalene | 21.84 | C15H24 | 483-76-1 | 75.42 | 1442 | 0.12 |
| 43 | α-Guaiene | 21.91 | C15H24 | 3691-12-1 | 90.65 | 1444 | 0.58 |
| 44 | cis-Calamenene | 22.23 | C15H22 | 72937-55-4 | 69.79 | 1457 | 0.18 |
| 45 | α-Humulene | 22.31 | C15H24 | 6753-98-6 | 97.41 | 1460 | 11.23 |
| 46 | Bicyclosesquiphellandrene | 22.54 | C15H24 | 54324-03-7 | 91.10 | 1469 | 0.61 |
| 47 | 2, 6-Bis (1, 1-dimethylethyl)-2, 5-cyclohexadiene-1, 4-dione | 22.61 | C14H20O2 | 719-22-2 | 91.04 | 1472 | 0.11 |
| 48 | cis-Muurola-4(15), 5-diene | 22.70 | C15H24 | 157477-72-0 | 68.22 | 1476 | 0.14 |
| 49 | (1α, 4aβ, 8aα)- (+/-)-1, 2, 4a, 5, 8, 8a-Hexahydro-4, 7-1-(1-methylethyl)-naphthalene | 22.86 | C15H24 | 5951-61-1 | 89.26 | 1482 | 0.75 |
| 50 | β-Guaiene | 23.00 | C15H24 | 88-84-6 | 93.97 | 1487 | 4.09 |
| 51 | [4aR-(4aα, 7α, 8aβ)]-Decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-naphthalene | 23.13 | C15H24 | 17066-67-0 | 87.43 | 1493 | 2.13 |
| 52 | 2-Isopropenyl-4a, 8-dimethyl-1, 2, 3, 4, 4a, 5, 6, 8a-octahydronaphthalene | 23.35 | C15H24 | 1000193-57-0 | 90.13 | 1501 | 2.78 |
| 53 | [1S-(1α, 7α, 8aβ)]-1, 2, 3, 5, 6, 7, 8,8a-octahydro-1,4-dimethyl-7-(1-methylethenyl)-azulene | 23.60 | C15H24 | 3691-11-0 | 89.20 | 1512 | 0.70 |
| 54 | Di-tert-butylphenol | 23.69 | C14H22O | 96-76-4 | 93.23 | 1515 | 20.11 |
| 55 | 1, 2, 3, 4, 4a, 5, 6, 8a-Octahydro-7-methyl-4-methylene-naphthalene | 23.80 | C15H24 | 39029-41-9 | 76.36 | 1520 | 0.37 |
| 56 | Selina-3, 7(11)-diene | 23.90 | C15H24 | 6813-21-4 | 54.29 | 1525 | 0.12 |
| 57 | (1S-cis)-1, 2, 3, 5, 6, 8a-Hexahydro-4, 7-dimethyl-1-(1-methylethyl)-naphthalene | 24.02 | C15H24 | 483-76-1 | 90.16 | 1530 | 0.90 |
| 58 | Epizonarene | 24.37 | C15H24 | 41702-63-0 | 63.48 | 1544 | 0.16 |
| 59 | Caryophyllene oxide | 24.74 | C15H24O | 1139-30-6 | 59.71 | 1560 | 0.18 |
| 60 | Patchoulene | 24.84 | C15H26 | 25491-20-7 | 85.19 | 1565 | 0.34 |
| 61 | Dehydro-aromadendrene | 25.32 | C15H22 | 85.63 | 1585 | 0.78 | |
| 62 | β-Vatirenene | 25.41 | C15H22 | 27840-40-0 | 72.71 | 1588 | 0.35 |
| 63 | Aristol-1(10)-en-9-ol | 25.47 | C15H24O | 1372763-27-3 | 81.55 | 1591 | 0.54 |
| 64 | (8R, 8aS)-8, 8a-Dimethyl-2-(propan-2-ylidene)-1, 2, 3, 7, 8, 8a-hexahydronaphthalene | 25.57 | C15H22 | 27840-40-0 | 69.23 | 1595 | 0.15 |
| 65 | Salvial-4(14)-en-1-one | 25.73 | C15H24O | 73809-82-2 | 66.55 | 1602 | 0.31 |
| 66 | 1, 3-Bis-(2-cyclopropyl,2-methylcyclopropyl)-but-2-en-1-one | 25.85 | C18H26O | 72.68 | 1607 | 0.11 | |
| 67 | [3R-(3α, 3aβ, 7β, 8aα)]-2, 3, 4, 7, 8, 8a-Hexahydro-3, 6, 8, 8-tetramethyl-1H-3a, 7-methanoazulene | 25.90 | C15H24 | 469-61-4 | 74.34 | 1610 | 0.19 |
| 68 | (+)-Epi-, β-santalyl acetate | 26.09 | C17H26O2 | 41414-75-9 | 75.95 | 1617 | 0.64 |
| 69 | (1S, 7S, 8aR)-1, 8a-Dimethyl-7-(prop-1-en-2-yl)-1, 2, 3, 7, 8, 8a-hexahydronaphthalene | 26.60 | C15H22 | 190327-38-9 | 61.30 | 1640 | 0.11 |
| 70 | 4a, 5-Dimethyl-3-(prop-1-en-2-yl)-1, 2, 3, 4, 4a, 5, 6, 7-octahydronaphthalen-1-ol | 26.69 | C15H24O | 61847-19-6 | 67.23 | 1645 | 0.19 |
| 71 | 1-Isopropyl-4, 7-dimethyl-1, 2, 3, 4, 5, 6-hexahydronaphthalene | 26.89 | C15H24 | 16729-00-3 | 59.31 | 1649 | 0.12 |
| 72 | (4aR-trans)-Decahydro-4a-methyl-1-methylene-7-(1-methylethylidene)-naphthalene | 27.09 | C15H24 | 515-17-3 | 80.30 | 1662 | 0.62 |
| 73 | trans-Valerenyl acetate | 27.14 | C17H26O2 | 101527-74-6 | 72.98 | 1665 | 0.21 |
| 74 | (E)-2-((8R, 8aS)-8, 8a-Dimethyl-3, 4, 6, 7, 8, 8a-hexahydronaphthalen-2 (1H)-ylidene) propyl formate | 27.41 | C16H24O2 | 352457-47-7 | 54.29 | 1679 | 0.72 |
| 75 | cis-α-Copaene-8-ol | 27.79 | C15H24O | 58569-25-8 | 55.35 | 1694 | 0.29 |
| 76 | Heptadecane | 27.98 | C17H36 | 629-78-7 | 68.57 | 1702 | 0.19 |
| 77 | (E)-1, 3, 3-trimethyl-2-(3-methyl-2-methylene-3-butenylidene)-cyclohexanol | 28.27 | C15H24O | 69296-93-1 | 50.18 | 1716 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liang, X. Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix. Foods 2019, 8, 205. https://doi.org/10.3390/foods8060205
Zhang W, Liang X. Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix. Foods. 2019; 8(6):205. https://doi.org/10.3390/foods8060205
Chicago/Turabian StyleZhang, Wei, and Xianrui Liang. 2019. "Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix" Foods 8, no. 6: 205. https://doi.org/10.3390/foods8060205
APA StyleZhang, W., & Liang, X. (2019). Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix. Foods, 8(6), 205. https://doi.org/10.3390/foods8060205





