The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Tea and Rosemary Extracts
2.2. Determination of the Antioxidant Capacity of Extracts
2.2.1. DPPH Radical Scavenging Activity
2.2.2. β-carotene Bleaching Assay
2.2.3. Total Phenolics Content (TPC)
2.2.4. Total Flavonoids Content
2.3. Development of the WP Active Film
2.4. Effectiveness of the WP Active Film
2.4.1. Sample Preparation
2.4.2. Fat Extraction
2.4.3. Fatty Acids Profile
2.4.4. Peroxide Value
2.4.5. p-Anisidine Value
2.4.6. TBARS Assay
2.4.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity
3.1.1. DPPH Radical Scavenging Activity and β-carotene Bleaching Assays
3.1.2. Determination of Total Phenolics and Flavonoids Content
3.2. Active Film Development
Glycerol and Green Tea Extract
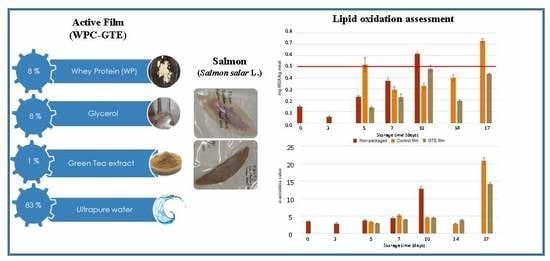
3.3. Lipid Oxidation Assessment
3.3.1. Determination of Fatty Acids
3.3.2. Peroxide Value
3.3.3. Determination of p-anisidine Value
3.3.4. TBARS Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro dos Santos, R. Development of protein active film incorporated with combination of essential oils: characterization and effectiveness. Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brasil, 2016. [Google Scholar]
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk Proteins for Edible Films and Coatings. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Tomasula, P.M. Using dairy ingredients to produce edible films and biodegradable packaging materials. In Dairy-Derived Ingredients; Elsevier: Cambridge, UK, 2009; pp. 589–624. ISBN 9781845694654. [Google Scholar]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.P.L.F.; Bertan, L.C.; De Rensis, C.M.V.B.; Bilck, A.P.; Vianna, P.C.B. Whey protein-based films incorporated with oregano essential oil. Polímeros 2017, 27, 158–164. [Google Scholar] [CrossRef]
- Hong-jiang, W.; Cheng, S.; Li-qiang, H. Preparation and Properties of Whey Protein Packaging Film. In Proceedings of the 17th IAPRI World Conference on Packaging, Tianjin, China, 12–15 October 2010; pp. 259–264. [Google Scholar]
- Ribeiro-Santos, R.; Motta, J.F.G.; Melo, N.R.; Costa, B.S.; Gonçalves, S.M. Elaboration of active films with whey protein isolate and concentrate. Int. Food Res. J. 2018, 25, 820–825. [Google Scholar]
- Marturano, V.; Bizzarro, V.; Ambrogi, V.; Cutignano, A.; Tommonaro, G.; Abbamondi, G.R.; Giamberini, M.; Tylkowski, B.; Carfagna, C.; Cerruti, P. Light-Responsive Nanocapsule-Coated Polymer Films for Antimicrobial Active Packaging. Polymers 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; Haas Costa, T.M.; de Oliveira Rios, A.; Hickmann Flôres, S. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crops Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- European Food Safety Authority. Use of rosemary extracts as a food additive—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 721, 1–29. [Google Scholar]
- Kim, H.-S.; Quon, M.J.; Kim, J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- López-de-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Kristam, P.; Eswarapragada, N.M.; Bandi, E.R.; Tumati, S.R. Evaluation of edible polymer coatings enriched with green tea extract on quality of chicken nuggets. Vet. World 2016, 9, 685–692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control. 2018, 92, 37–46. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT-Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH· Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N. Highly hydroxylated or γ-cyclodextrin-bicapped water-soluble derivative of fullerene: The antioxidant ability assessed by electron spin resonance method and β-carotene bleaching assay. Bioorg. Med. Chem. Lett. 2009, 19, 5293–5296. [Google Scholar] [CrossRef] [PubMed]
- Phan-Thi, H.; Durand, P.; Prost, M.; Prost, E.; Waché, Y. Effect of heat-processing on the antioxidant and prooxidant activities of β-carotene from natural and synthetic origins on red blood cells. Food Chem. 2016, 190, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Franco, D.; Sineiro, J.; Domı́nguez, H.; Núñez, M.J.; Lema, J.M. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res. Int. 2001, 34, 103–109. [Google Scholar] [CrossRef]
- Cruz, J.M.; Conde, E.; Domínguez, H.; Parajó, J.C. Thermal stability of antioxidants obtained from wood and industrial wastes. Food Chem. 2007, 100, 1059–1064. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- ISO 5509:2000. Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. 2000. Available online: https://www.iso.org/standard/11560.html (accessed on 8 August 2019).
- The American Oil Chemists’ Society. AOCS Official Method Cd 8b-90. Peroxide Value, Acetic Acid, Isooctane Method; The American Oil Chemists’ Society: Urbana, IL, USA, 1989. [Google Scholar]
- BS 684-2.24 Methods of Analysis of Fats and Fatty Oils. Other methods. Determination of Anisidine Value. British Standards Institution, November 1998. Available online: https://doi.org/10.3403/00196121U (accessed on 8 August 2019).
- Miller, D. Food Chemistry: A Laboratory Manual, 2nd ed.; Wiley: New York, NY, USA, 1998; ISBN 978-0471175438. [Google Scholar]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Pereira, D.; Pinheiro, R.S.; Heldt, L.F.S.; de Moura, C.; Bianchin, M.; Almeida, J.d.F.; dos Reis, A.S.; Ribeiro, I.S.; Haminiuk, C.W.I.; Carpes, S.T. Rosemary as natural antioxidant to prevent oxidation in chicken burgers. Food Sci. Technol. 2017, 37, 17–23. [Google Scholar] [CrossRef]
- Reis, A.R.d.S. Estudo de compostos bioactivos e vitaminas de plantas aromáticas e sua aplicação em embalagens alimentares activas. MSc Thesis, Faculty of Sciences, University of Lisbon, Lisbon, Portugal, 2014. [Google Scholar]
- Rivelli, D.P. Biodisponibilidade, distribuição tecidual e atividade antioxidante do extrato hidroetanólico de Ilex paraguariensis hidrolisado e não hidrolisado. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2011. [Google Scholar]
- Kmiecik, D.; Gramza-Michałowska, A.; Korczak, J. Anti-polymerization activity of tea and fruits extracts during rapeseed oil heating. Food Chem. 2018, 239, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total Phenolics and Antioxidant Activities of Fenugreek, Green Tea, Black Tea, Grape Seed, Ginger, Rosemary, Gotu Kola, and Ginkgo Extracts, Vitamin E, and tert-Butylhydroquinone. J. Agric. Food Chem. 2004, 52, 5183–5186. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Ponce, A.G.; Alvarez, V.A. Nano-clays from natural and modified montmorillonite with and without added blueberry extract for active and intelligent food nanopackaging materials. Mater. Chem. Phys. 2017, 194, 283–292. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effects of plasticizers, pH and heating of film-forming solution on the properties of pea protein isolate films. J. Food Eng. 2011, 105, 295–305. [Google Scholar] [CrossRef]
- Cascant, M.M.; Breil, C.; Fabiano-Tixier, A.S.; Chemat, F.; Garrigues, S.; de la Guardia, M. Determination of fatty acids and lipid classes in salmon oil by near infrared spectroscopy. Food Chem. 2018, 239, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Kezić, N.; Bolanča, V. Optimization of the GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 122, 285–291. [Google Scholar] [CrossRef]
- National Institute of Health Dr. Ricardo Jorge (INSA). Tabela da Composição de Alimentos. Available online: http://portfir.insa.pt/foodcomp/food?434 (accessed on 30 November 2018).
- USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 30 November 2018).
- Mehta, B.M.; Darji, V.B.; Aparnathi, K.D. Comparison of five analytical methods for the determination of peroxide value in oxidized ghee. Food Chem. 2015, 185, 449–453. [Google Scholar] [CrossRef]
- Dermiş, S.; Can, S.; Doğru, B. Determination of Peroxide Values of Some Fixed Oils by Using the mFOX Method. Spectrosc. Lett. 2012, 45, 359–363. [Google Scholar] [CrossRef]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation—xylenol orange method: A review of the factors that influence the method’s performance. Anal. Biochem. 2008, 377, 1–15. [Google Scholar] [CrossRef]
- Yang, X.; Boyle, R.A. Sensory Evaluation of Oils/Fats and Oil/Fat–Based Foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier: Kalamazoo, MI, USA, 2016; pp. 157–185. ISBN 9781630670566. [Google Scholar]
- Guo, Q.; Gao, S.; Sun, Y.; Gao, Y.; Wang, X.; Zhang, Z. Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Ind. Crops Prod. 2016, 94, 82–88. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K.; Dasgupta, A.; Klein, K. Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier: San Diego, CA, USA, 2014; pp. 19–40. [Google Scholar]
- Osawa, C.C.; De Felício, P.E.; Gonçalves, L.A.G. Teste de TBA aplicado a carnes e derivados: Métodos tradicionais, modificados e alternativos. Quim. Nova 2005, 28, 655–663. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Bindu, J.; Sivaraman, G.K.; Venkateshwarlu, G.; Ravishankar, C.N. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Özalp Özen, B.; Soyer, A. Effect of plant extracts on lipid and protein oxidation of mackerel (Scomber scombrus) mince during frozen storage. J. Food Sci. Technol. 2018, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]



| Concentration (mg/mL) | DPPH Assay | |||
|---|---|---|---|---|
| Inhibition Percentage (%) | μg TE/mL | |||
| GTE | RE | GTE | RE | |
| 0.1 | 51.43 ± 1.98 a | 13.36 ± 0.24 b | 83.74 ± 3.73 A | 22.02 ± 0.37 B |
| 0.2 | 86.62 ± 0.12 c | 24.43 ± 0.38 d | 133.41 ± 7.03 C | 39.47 ± 0.59 D |
| Phenolic Content (mg GAE/g Extract) | Flavonoid Content (mg ECE/g Extract) | ||
|---|---|---|---|
| GTE | RE | GTE | RE |
| 443.55 ± 10.00 a | 190.23 ± 2.97 b | 119.81 ± 11.70 A | 118.69 ± 5.09 B |
| Films | GTE (%, w/w) | Glycerol (%, w/w) |
|---|---|---|
| F1 | 2 | 5 |
| F2 | 1 | 5 |
| F3 | 2 | 8 |
| F4 | 1 | 8 |
| Nutrient | Unit | Wild Salmon | Farmed Salmon | Salmon (Salmon salar L.) |
|---|---|---|---|---|
| Water | g | 68.5 | 65.9 | 60.5 |
| Energy | kcal | 142 | 208 | 262 |
| Protein | g | 19.8 | 20.4 | 16.2 |
| Total lipid | g | 6.3 | 13.4 | 21.9 |
| Ash | g | 2.5 | 1.1 | 1.3 |
| Carbohydrate (by difference) | g | 0 | 0 | 0 |
| Fiber (total dietary) | g | 0 | 0 | 0 |
| Minerals | ||||
| Calcium (Ca) | mg | 12 | 9 | 12 |
| Iron (Fe) | mg | 0.8 | 0.3 | 0.5 |
| Magnesium (Mg) | mg | 29 | 27 | 23 |
| Phosphorus (P) | mg | 200 | 240 | 210 |
| Potassium (K) | mg | 490 | 363 | 300 |
| Sodium (Na) | mg | 44 | 59 | 38 |
| Zinc (Zn) | mg | 0.6 | 0.4 | 0.5 |
| Copper (Cu) | mg | 0.3 | 0.05 | - |
| Manganese (Mn) | mg | 0.02 | 0.01 | - |
| Selenium (Se) | μg | 36.5 | 24 | - |
| Vitamins | ||||
| Thiamin | mg | 0.2 | 0.2 | 0.18 |
| Riboflavin | mg | 0.4 | 0.2 | 0.04 |
| Niacin | mg | 7.9 | 8.7 | 3.6 |
| Pantothenic acid | mg | 1.7 | 1.5 | - |
| Vitamin B-6 | mg | 0.8 | 0.6 | 0.45 |
| Folate (DFE) | μg | 25 | 26 | 10 |
| Vitamin B-12 | μg | 3.2 | 3.2 | 1.9 |
| Vitamin A (RAE) | μg | 12 | 58 | 33 |
| Retinol | μg | 12 | 58 | - |
| Vitamin A (IU) | IU | 40 | 193 | - |
| Lipids | ||||
| Fatty acids (total saturated) | g | 1.0 | 3.1 | 4.2 |
| 14:0 | g | 0.1 | 0.6 | - |
| 16:0 | g | 0.6 | 1.9 | - |
| 18:0 | g | 0.2 | 0.5 | - |
| Fatty acids (total monounsaturated) | g | 2.1 | 3.8 | 10 |
| 16:1 undifferentiated | g | 0.3 | 0.8 | - |
| 18:1 undifferentiated | g | 1.4 | 2.7 | - |
| 20:1 | g | 0.2 | 0.3 | - |
| 22:1 undifferentiated | g | 0.3 | 0 | - |
| Fatty acids (total polyunsaturated) | g | 2.5 | 3.9 | 5.1 |
| 18:2 undifferentiated | g | 0.2 | 0.9 | - |
| 18:3 undifferentiated | g | 0.3 | 0.2 | - |
| 18:4 | g | 0.1 | 0.1 | - |
| 20:4 undifferentiated | g | 0.3 | 0.1 | - |
| 20:5 n-3 (EPA) | g | 0.3 | 0.9 | - |
| 22:5 n-3 (DPA) | g | 0.3 | 0.4 | - |
| 22:6 n-3 (DHA) | g | 1.1 | 1.1 | - |
| Cholesterol | mg | 55 | 55 | 40 |
| Reference | - | USDA | USDA | TCAP |
| Fatty Acids | Percentage of Fatty Acids in g/100 g of Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-packaged Fresh Salmon | Salmon Packed with Control Film | Salmon Packed with Active Film | ||||||
| 0 Days | 5 Days | 7 Days | 12 Days | 5 Days | 7 Days | 5 Days | 7 Days | |
| Saturated | 3.7 | 3.7 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Monounsaturated | 9.7 | 9.5 | 9.7 | 9.7 | 9.7 | 9.8 | 9.6 | 9.7 |
| Polyunsaturated | 7.5 | 7.6 | 7.6 | 7.6 | 7.6 | 7.5 | 7.6 | 7.6 |
| Polyunsaturated of which n3 | 1.3 | 1.7 | 1.4 | 1.4 | 1.5 | 1.3 | 1.5 | 1.5 |
| Polyunsaturated of which n6 | 6.0 | 5.8 | 6.0 | 6.0 | 5.9 | 5.9 | 5.9 | 5.9 |
| trans | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, F.V.R.; Andrade, M.A.; Sanches Silva, A.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods 2019, 8, 327. https://doi.org/10.3390/foods8080327
Castro FVR, Andrade MA, Sanches Silva A, Vaz MF, Vilarinho F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods. 2019; 8(8):327. https://doi.org/10.3390/foods8080327
Chicago/Turabian StyleCastro, Frederico V. R., Mariana A. Andrade, Ana Sanches Silva, Maria Fátima Vaz, and Fernanda Vilarinho. 2019. "The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.)" Foods 8, no. 8: 327. https://doi.org/10.3390/foods8080327
APA StyleCastro, F. V. R., Andrade, M. A., Sanches Silva, A., Vaz, M. F., & Vilarinho, F. (2019). The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods, 8(8), 327. https://doi.org/10.3390/foods8080327