Analysis of the Acid Detergent Fibre Content in Turnip Greens and Turnip Tops (Brassica rapa L. Subsp. rapa) by Means of Near-Infrared Reflectance †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Acid Detergent Fibre
2.3. Development of NIRS Equations
2.4. Equation Validation
2.4.1. Cross Validation
2.4.2. External Validation
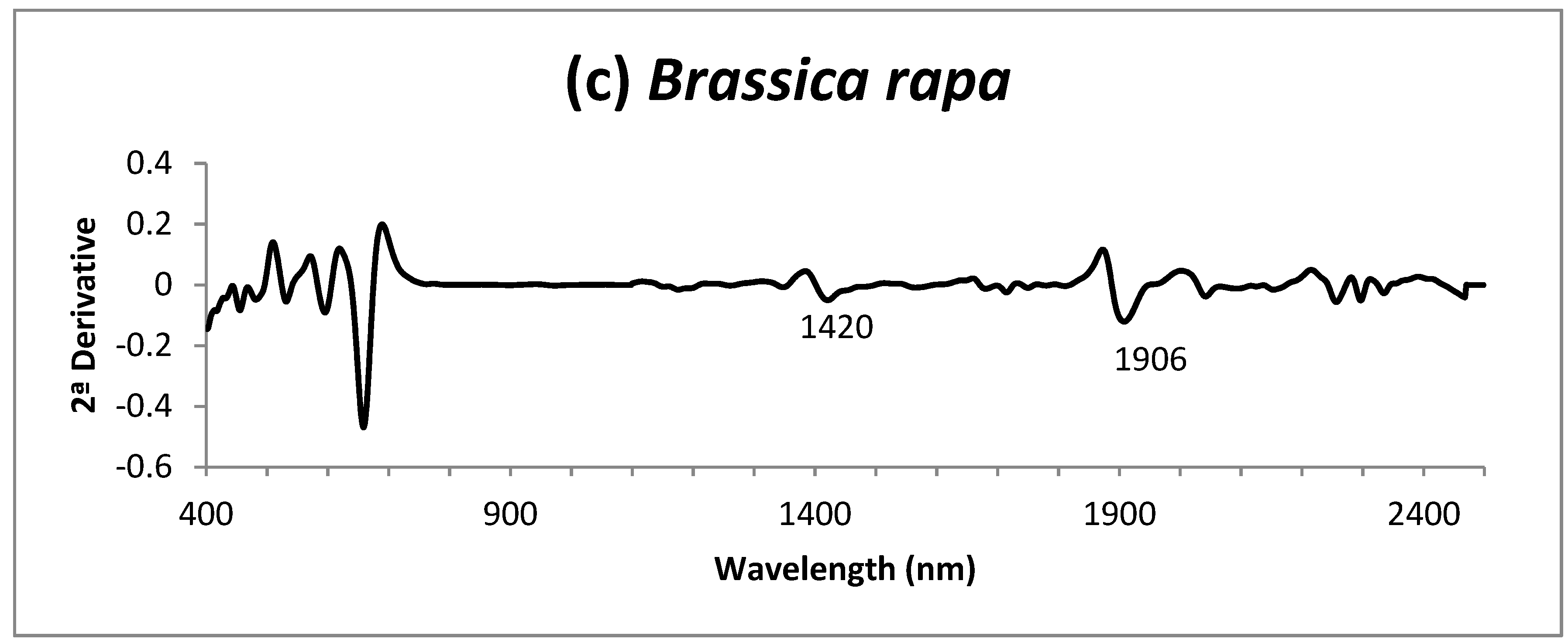
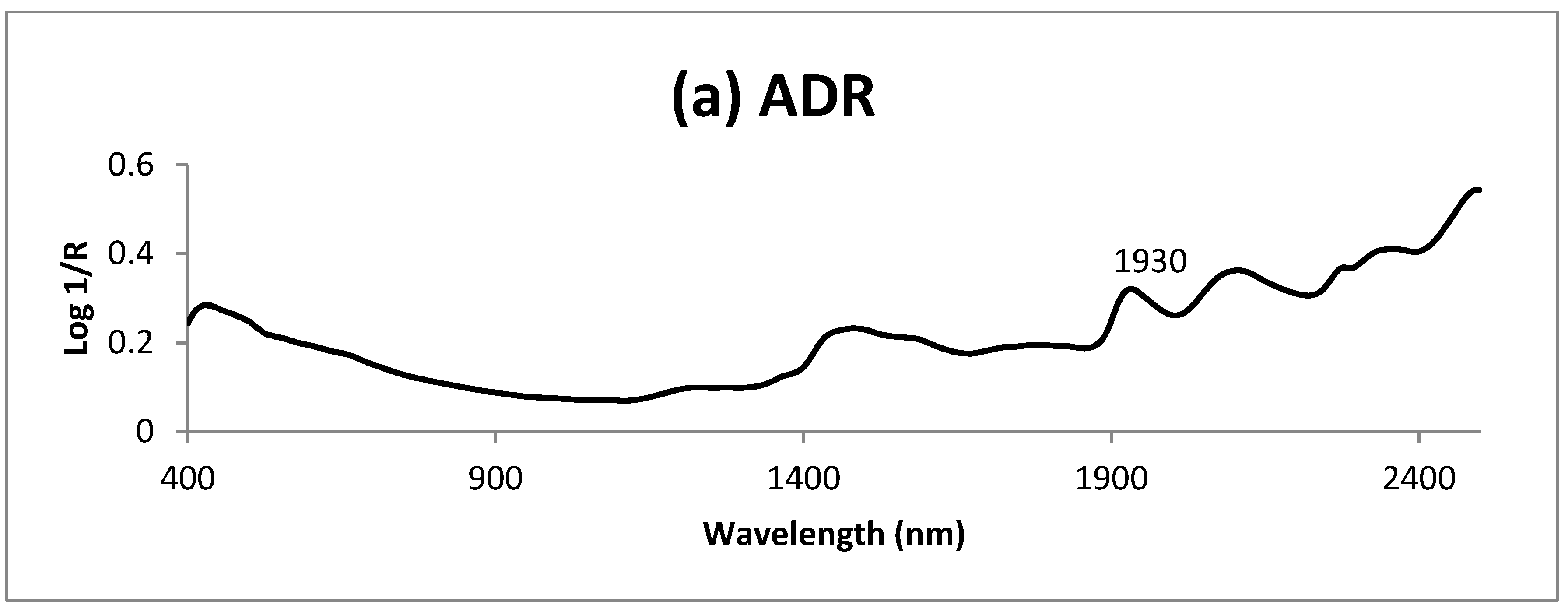
3. Results and Discussion
3.1. ADF Reference Analysis in Samples of Turnip Tops and Turnip Greens
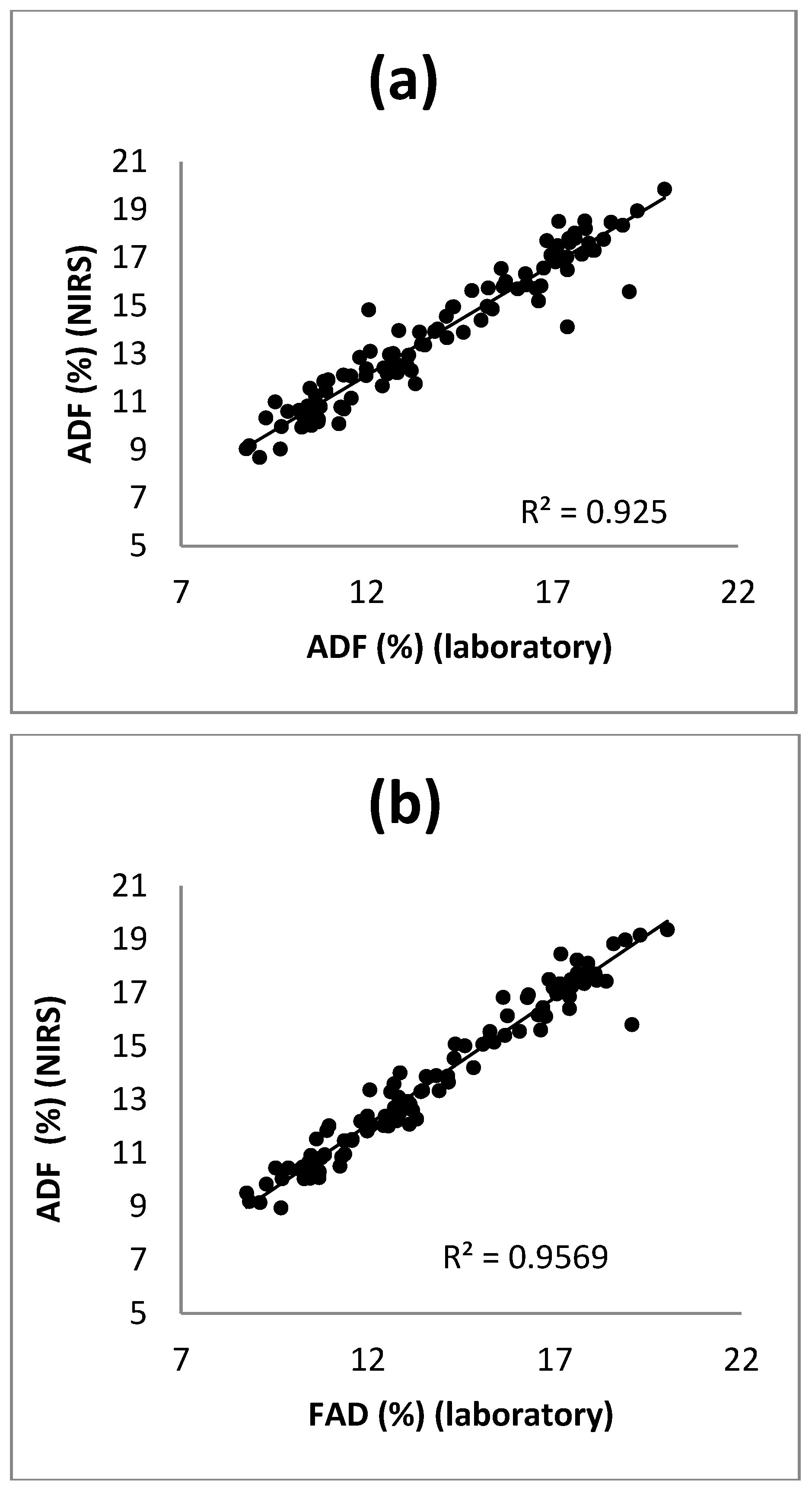
3.2. Calibration and Validation
3.1.1. Cross Validation
3.1.2. External Validation
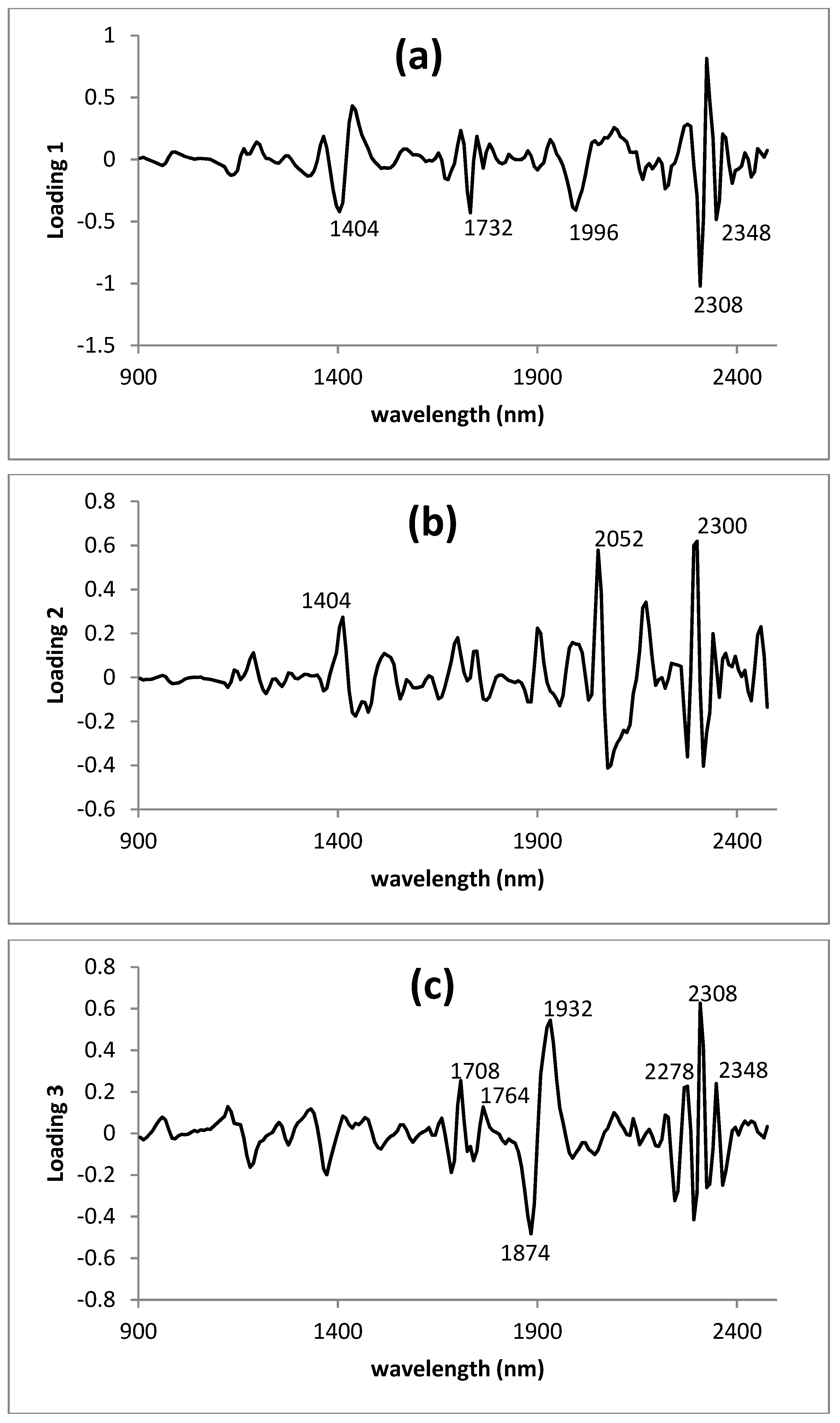
3.3. Modified Partial Least Squares Loadings of the Lyophilized Green Parts Model
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Campo, C. Brassica Crops and Wild Allies. In Morphology and Morpho-Taxonomy of the Tribe Brassiceae; Scientific Societies Press: Tokyo, Japan, 1980. [Google Scholar]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods:bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Prakash, O. Food and Drinks in Ancient India; Munshi Ram Manohar Lal: Delhi, India, 1961; pp. 165–168. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method 978.10. Fiber (Crude) in animal feed Fritted glass crucible method. In AOAC Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1995; Volume 1, pp. 20–21. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis; apparatus, reagents, procedures and some applications. In USDA-ARS Agricultrual; Handbook no 379; U.S. Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Oh, H.K.; Baumgardt, B.R.; Scholl, J.M. Evaluation of forages in the laboratory. V. Comparison of chemical analysis, solubility tests, and in vitro fermentation. J. Dairy Sci. 1966, 49, 850–855. [Google Scholar] [CrossRef]
- Archer, K.A.; Decker, A.M. Relationship between fibrous components and in vitro dry matter digestibility of autumn-saved grasses. Agron. J. 1997, 69, 610–612. [Google Scholar] [CrossRef]
- Hill, R.R.; Barnes, R.F. Genetic Variability for Chemical Composition of Alfalfa. II. Yield and Traits Associated with Digestibility. Crop Sci. 1977, 17, 948–952. [Google Scholar] [CrossRef]
- Soh, A.C.; Frakes, R.V.; Chilcote, D.O.; Sleper, D.A. Genetic variation in acid detergent fiber, neutral detergent fiber, hemicellulose, crude protein, and their relationship with in vitro dry matter digestibility in tall fescue. Crop Sci. 1984, 24, 721–727. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B. Analysis of Forages and Fibrous Foods; Cornell University Publication: Ithaca, NY, USA, 1985; Volume 165. [Google Scholar]
- Scotter, C. Use of near infrared spectroscopy in the food industry with particular reference to its applications to on/in-line food processes. Food Control 1990, 1, 142–149. [Google Scholar] [CrossRef]
- Bochereau, L.; Bourgine, P.; Palagos, B. A method for prediction by combining data analysis and neural networks: Application to prediction of apple quality using near infra-red spectra. J. Agric. Eng. Res. 1992, 51, 207–216. [Google Scholar] [CrossRef]
- Font, R.; Wittkop, B.; Badani, A.G.; Del Río-Celestino, M.; Friedt, W.; Lühs, W.; De Haro-Bailón, A. The measurements of acid detergent fiber in rapeseed by visible and near-infrared spectroscopy. Plant Breed. 2005, 124, 410–412. [Google Scholar] [CrossRef]
- Font, R.; Del Río, M.; De Haro-Bailón, A. The use of near-infrared spectroscopy (NIRS) in the study of seed quality components in plant breeding programs. Ind. Crops Prod. 2006, 24, 307–313. [Google Scholar] [CrossRef]
- Cozzolino, D.; Moron, A. Exploring the use of near infrared reflectance spectroscopy (NIRS) to predict trace minerals in legumes. Anim. Feed Sci. Technol. 2004, 111, 161–173. [Google Scholar] [CrossRef]
- Sinelli, N.; Spinardi, A.; Di Egidio, V.; Mignani, I.; Casiraghi, E. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharv. Biol. Technol. 2008, 50, 31. [Google Scholar] [CrossRef]
- Davey, M.W.; Saeys, W.; Hof, E.; Ramon, H.; Swennen, R.L.; Keulemans, J. Application of visible and nearinfrared reflectance spectroscopy (Vis/NIRS) to determine carotenoid contents in banana (Musa spp.) fruit pulp. J. Agric. Food Chem. 2009, 57, 1742. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, T.; Wold, J.P.; Pedersen, M.B.; Liland, K.H.; Ballance, S.; Knutsen, S.H.; Rukke, E.O.; Afseth, N.K. Towards on-line prediction of dry matter content in whole unpeeled potatoes using near-infrared spectroscopy. Talanta 2015, 143, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hell, J.; Prückler, M.; Danner, L.; Henniges, U.; Apprich, S.; Rosenau, T.; Kneifel, W.; Böhmdorfer, S. A comparison between near-infrared (NIR) and mid-infrared (ATR-FTIR) spectroscopy for the multivariate determination of compositional properties in wheat bran samples. Food Control 2016, 60, 365–369. [Google Scholar] [CrossRef]
- Guo, Y.; Ni, Y.; Kokot, S. Evaluation of chemical components and properties of the jujube fruit using near infrared spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 79–86. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Martínez-Marín, A.L.; Polvillo, O.; Fernández-Cabanás, V.M.; Carrizosa, J.; Urrutia, B.; Serradilla, J.M. Near Infrared Spectroscopy (NIRS) for the determination of the milk fat fatty acid profile of goats. Food Chem. 2016, 190, 244–252. [Google Scholar] [CrossRef]
- Panford, A.; Williams, P.C.; Man, J.M. Analysis of oilseeds for protein, oil, fiber and moisture by near-infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 1988, 65, 1627–1634. [Google Scholar] [CrossRef]
- Michalski, K.; Ochodzki, P.; Cicha, B. Determination of fiber, sulphur amino acids and lysine in oilseed rape by NIT. In Making Light Work: Advances in Near Infrared Spectroscopy; Murray, I., Cowe, I.A., Eds.; VCH Weinheim: New York, NY, USA, 1992; pp. 333–335. [Google Scholar]
- Font, R.; Del Río, M.; Fernández, J.M.; De Haro-Bailón, A. Acid Detergent Fiber Analysis in Oilseed Brassicas by Near-Infrared Spectroscopy. J. Agric. Food Chem. 2003, 51, 2917–2922. [Google Scholar] [CrossRef] [PubMed]
- Dimov, Z.; Suprianto, E.; Hermann, F. Möllers. Genetic variation for seed hull and fiber content in a collection of European winter oilseed rape material (Brassica napus L.) and development of NIRS calibrations. Plant Breed. 2012, 131, 361–368. [Google Scholar] [CrossRef]
- Wittkop, B.; Snowdon, R.; Friedt, W. New NIRS Calibrations for Fiber Fractions Reveal Broad Genetic Variation in Brassica napus Seed Quality. J. Agric. Food Chem. 2012, 60, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Kakani, V.G.; Ge, J.; Mosali, J. Rapid assessment of bioenergy feedstock quality by near infrared reflectance spectroscopy. Agron. J. 2013, 105, 1487–1497. [Google Scholar] [CrossRef]
- Daniel, J.L.P.; Capelesso, A.; Cabezas-Garcia, E.H.; Zopollatto, M.; Santos, M.C.; Huhtanen, P.; Nussio, L.G. Fiber digestion potential in sugarcane across the harvesting window. Grass Forage Sci. 2014, 69, 176–181. [Google Scholar] [CrossRef]
- Krizsan, S.J.; Rinne, M.; Nyholm, L.; Huhtanen, P. New recommendations for the ruminal in situ determination of indigestible neutral detergent fiber. Anim. Feed Sci. Technol. 2015, 205, 31–41. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Population definition, sample selection, and calibration procedures for near infrared reflectance spectroscopy. Crop. Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI way. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P., Eds.; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]
- Martens, H.; Naes, T. Multivariate Calibration; John Wiley and Sons: Chichester, UK, 1989. [Google Scholar]
- Hruschka, W.R. Data Analysis Wavelength Selection Methods. In Near Infrared Technology in the Agricultural, Food Industries; Williams, P.C., Norris, K.H., Eds.; American Association of Cereal Chemist: St. Paul, MN, USA, 2001; pp. 35–55. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of Near-Infrared Analysis; Burns, D.A., Ciurczak, E., Eds.; Dekker Inc.: New York, NY, USA, 1992; pp. 383–431. [Google Scholar]
- Williams, P.C. Implementation of Near-Infrared technology. In Near Infrared Technology in the Agricultural, Food Industries; Williams, P.C., Norris, K.H., Eds.; American Association of Cereal Chemist: St. Paul, MN, USA, 2001; pp. 145–169. [Google Scholar]
- Williams, P.C.; Sobering, D.C. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P.C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Türk, M.; Albayrak, S.; Balabanli, C.; Yüksel, O. Effects of fertilization on root and leaf yields and quality of forage turnip (Brassica rapa L.). J. Food Agric. Environ. 2009, 7, 339–342. [Google Scholar]
- Francisco, M.; Velasco, P.; Lema, M.; Cartea, M.E. Genotypic and Environmental Effects on Agronomic and Nutritional Value of Brassica rapa. Agron. J. 2011, 103, 735–742. [Google Scholar] [CrossRef]
- Azam, A.; Khan, I.; Mahmood, A.; Hameed, A. Yield, chemical composition and nutritional quality responses of carrot, radish and turnip to elevated atmospheric carbon dioxide. J. Sci. Food Agric. 2013, 93, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Daszykowski, M.; Wrobel, M.S.; Czarnik-Matusewicz, H.; Walczak, B. Near-infrared reflectance spectroscopy and multivariate calibration techniques applied to modelling the crude protein, fiber and fat content in rapeseed meal. Analyst 2008, 133, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Petisco, C.; García-Criado, B.; Vázquez de Aldana, B.R.; Zabalgogeazcoa, I.; Mediavilla, S.; García-Ciudad, A. Use of near-infrared reflectance spectroscopy in predicting nitrogen, phosphorus and calcium contents in heterogeneous woody plant species. Anal. Bioanal. Chem. 2005, 382, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.G. Near Infrared Spectroscopy in Food Analysis; Longman Scientific and Technical: New York, NY, USA, 1986. [Google Scholar]
- Bagchi, T.B.; Sharma, S.; Chattopadhyay, K. Development of NIRS models to predict protein and amylose content of brown rice and proximate compositions of rice bran. Food Chem. 2016, 191, 21–27. [Google Scholar] [CrossRef]
- Petisco, C.; García-Criado, B.; Mediavilla, S.; Vázquez de Aldana, B.R.; Zabalgogeazcoa, I.; García-Ciudad, A. Near-infrared reflectance spectroscopy as a fast and non-destructive tool to predict foliar organic constituents of several woody species. Anal. Bioanal Chem. 2006, 386, 1823–1833. [Google Scholar] [CrossRef]
- Campo, L.; Monteagudo, A.B.; Salleres, B.; Castro, P.; Moreno-Gonzalez, J. NIRS determination of non-structural carbohydrates, water soluble carbohydrates and other nutritive quality traits in whole plant maize with wide range variability. Span. J. Agric. Res. 2013, 11, 463–471. [Google Scholar] [CrossRef]
- Nordheim, H.; Volden, H.; Fystro, G.; Lunnan, T. Prediction of in situ degradation characteristics of neutral detergent fiber (aNDF) in temperate grasses and red clover using near-infrared reflectance spectroscopy (NIRS). Anim. Feed Sci. Technol. 2007, 139, 92–108. [Google Scholar] [CrossRef]




| Plant Material | ADF (%) | ||
|---|---|---|---|
| Range | Mean | SD 1 | |
| Turnip greens (n = 63) | 8.55–15.27 | 11.53 | 1.54 |
| Turnip tops (n = 71) | 10.41-21.91 | 15.98 | 2.54 |
| Sample Groups | ADF (%) | ||
|---|---|---|---|
| Range | Mean | SD 1 | |
| Calibration set (n = 104) | 8.75–20.02 | 13.87 | 2.98 |
| Validation set (n = 26) | 8.55–18.81 | 13.67 | 3.01 |
| Calibration | Cross Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TM 1 | Range | Samples | Mean | SD 2 | SEC 3 | R24 | SECV 5 | RPDcv 6 | R2cv7 |
| 0, 0, 1, 1 | 8.75–20.02 | 101 | 13.81 | 2.95 | 0.86 | 0.91 | 1.07 | 2.77 | 0.87 |
| 1, 4, 4, 1 | 8.75–20.02 | 104 | 13.80 | 2.96 | 0.65 | 0.95 | 0.88 | 3.36 | 0.91 |
| 2, 5, 5, 2 | 8.75–20.02 | 103 | 13.82 | 2.95 | 0.56 | 0.96 | 0.89 | 3.33 | 0.91 |
| TM 1 | Range | Samples | Mean | SD 2 | SEP 3 | r2ev4 | RPDev 5 | RER 6 |
|---|---|---|---|---|---|---|---|---|
| 0, 0, 1, 1 | 8.55–18.81 | 26 | 13.67 | 3.13 | 1.14 | 0.87 | 2.75 | 9.00 |
| 1, 4, 4, 1 | 8.55–18.81 | 25 | 13.55 | 2.96 | 0.87 | 0.91 | 3.41 | 11.79 |
| 2, 5, 5, 2 | 8.55–18.81 | 25 | 13.55 | 2.89 | 0.93 | 0.91 | 3.10 | 11.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón-Cano, S.; Moreno-Rojas, R.; Jurado-Millán, A.M.; Cartea-González, M.E.; De Haro-Bailón, A. Analysis of the Acid Detergent Fibre Content in Turnip Greens and Turnip Tops (Brassica rapa L. Subsp. rapa) by Means of Near-Infrared Reflectance. Foods 2019, 8, 364. https://doi.org/10.3390/foods8090364
Obregón-Cano S, Moreno-Rojas R, Jurado-Millán AM, Cartea-González ME, De Haro-Bailón A. Analysis of the Acid Detergent Fibre Content in Turnip Greens and Turnip Tops (Brassica rapa L. Subsp. rapa) by Means of Near-Infrared Reflectance. Foods. 2019; 8(9):364. https://doi.org/10.3390/foods8090364
Chicago/Turabian StyleObregón-Cano, Sara, Rafael Moreno-Rojas, Ana María Jurado-Millán, María Elena Cartea-González, and Antonio De Haro-Bailón. 2019. "Analysis of the Acid Detergent Fibre Content in Turnip Greens and Turnip Tops (Brassica rapa L. Subsp. rapa) by Means of Near-Infrared Reflectance" Foods 8, no. 9: 364. https://doi.org/10.3390/foods8090364
APA StyleObregón-Cano, S., Moreno-Rojas, R., Jurado-Millán, A. M., Cartea-González, M. E., & De Haro-Bailón, A. (2019). Analysis of the Acid Detergent Fibre Content in Turnip Greens and Turnip Tops (Brassica rapa L. Subsp. rapa) by Means of Near-Infrared Reflectance. Foods, 8(9), 364. https://doi.org/10.3390/foods8090364





