Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olives Samples and Fermentation Procedure
2.2. Microbiological Analysis
2.3. Physicochemical Analysis
2.4. Color and Texture Analysis
2.5. Sensory Evaluation
- ❖
- Texture: 0 = soft, 5 = intermediate, 10 = coherent
- ❖
- Flavor: 0 = absence, 5 = moderate, 10 = strong
- ❖
- Salty: 0 = no, 5 = moderate, 10 = very much
- ❖
- Bitterness: 0 = No, 5 = moderate, 10 = high
- ❖
- Acidity: 0 = no, 5 = moderate, 10 = high
- ❖
- Off flavors: 0 = absence, 5 = moderate, 10 = strong
- ❖
- Overall acceptance: 0 = reject, 5 = moderate, 10 = strongly accept
2.6. Isolation of the Predominant Microflora
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analyses
3.2. Physicochemical Analyses
3.3. Firmness and Color Evolution of Olives
3.4. Sensory Evaluation
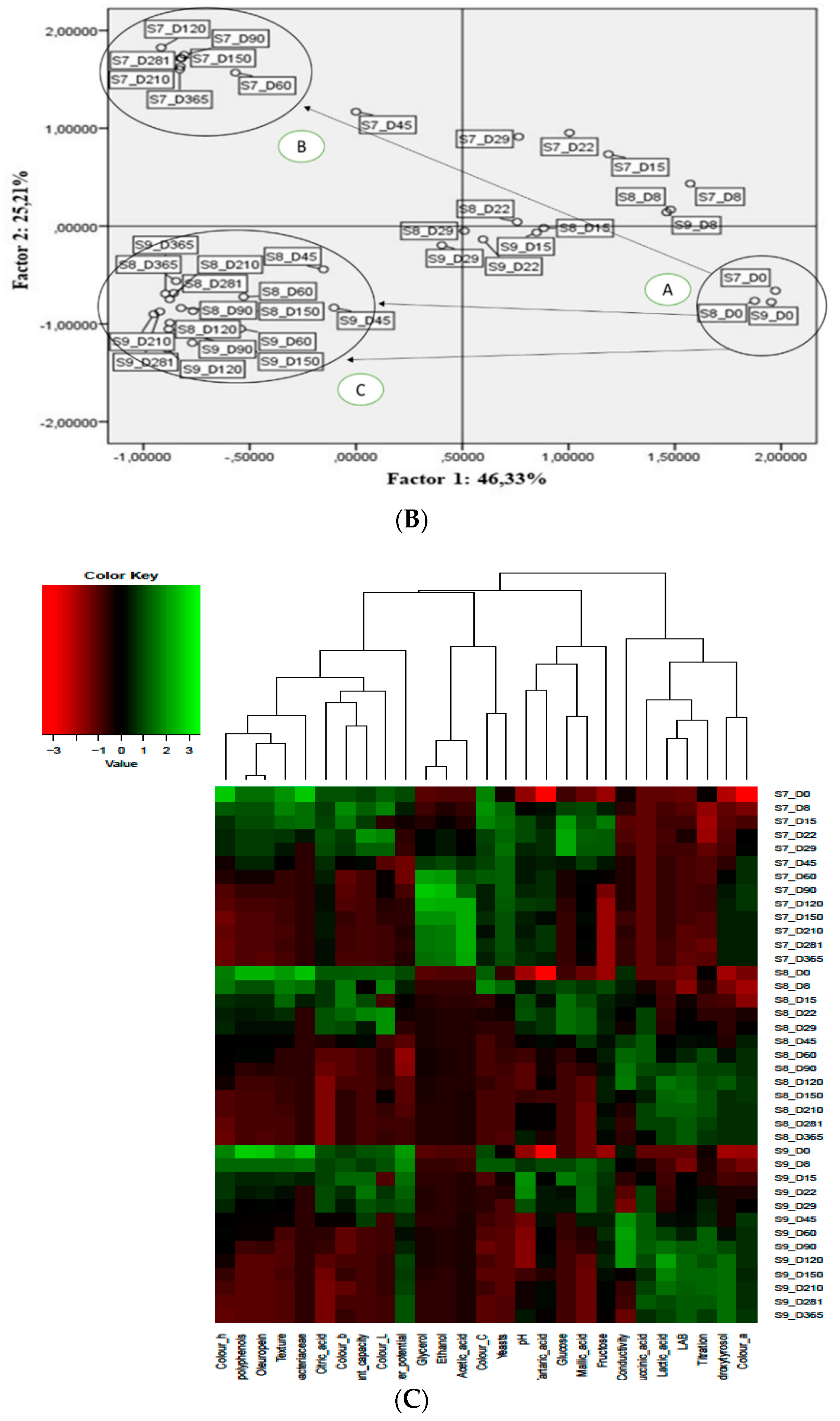
3.5. Multivariate Analysis
3.6. Detection of the Presence of the Starter Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- (IOC), I.O.O.C. Updates Series of World Statistics on Production, Imports, Exports and Consumption. 2018. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/production3_ang.pdf (accessed on 11 November 2019).
- Bleve, G.; Tufariello, M.; Durante, M.; Perbellini, E.; Ramires, F.A.; Grieco, F.; Cappello, M.S.; de Domenico, S.; Mita, G.; Tasioula-Margari, M.; et al. Physico-chemical and microbiological characterization of spontaneous fermentation of Cellina di Nardò and Leccino table olives. Front. Microbiol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Gómez, F.; Ruiz-Bellido, M.A.; Romero-Gil, V.; Benítez-Cabello, A.; Garrido-Fernández, A.; Arroyo-López, F.N. Microbiological and physicochemical changes in natural green heat-shocked Aloreña de Málaga table olives. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of starter cultures to table olive fermentation: An overview on the experimental studies. Front. Microbiol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campus, M.; Degirmencioglu, N.; Comunian, R. Technologies and trends to improve table olive quality and safety. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.; Panagou, E.; Nychas, G.-J. Table Olive Fermentation Using Starter Cultures with Multifunctional Potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Papadelli, M.; Zoumpopoulou, G.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Lytra, I.; Papadimitriou, K.; Tsakalidou, E. Evaluation of two lactic acid bacteria starter cultures for the fermentation of natural black table olives (Olea Europaea L cv Kalamon). Polish J. Microbiol. 2015, 64, 265–271. [Google Scholar] [CrossRef]
- Sorrentino, G.; Muzzalupo, I.; Muccilli, S.; Timpanaro, N.; Russo, M.P.; Guardo, M.; Rapisarda, P.; Romeo, F.V. New accessions of Italian table olives (Olea europaea): Characterization of genotypes and quality of brined products. Sci. Hortic. (Amsterdam) 2016, 213, 34–41. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.; Bozoudi, D.; Tsaltas, D. Yeast ecology of fermented table olives: A tool for biotechnlogical applications. In Yeast: Industrial Applications; IntechOpen: Rijeka, Croatia, 2017; pp. 135–152. [Google Scholar]
- Romeo, F.V. Microbiological Aspects of Table Olives. In Olive Germplasm: The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Kiai, H.; Hafidi, A. Chemical composition changes in four green olive cultivars during spontaneous fermentation. LWT Food Sci. Technol. 2014, 57, 663–670. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Schillinger, U.; Franz, C.M.A.P.; Nychas, G.J.E. Microbiological and biochemical profile of cv. Conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 2008, 25, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Durán-Quintana, M.C.; Garrido-Fernández, A. Fermentation profiles of Manzanilla-Aloreña cracked green table olives in different chloride salt mixtures. Food Microbiol. 2010, 27, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bleve, G.; Tufariello, M.; Durante, M.; Grieco, F.; Ramires, F.A.; Mita, G.; Tasioula-Margari, M.; Logrieco, A.F. Physico-chemical characterization of natural fermentation process of Conservolea and Kalamàta table olives and developement of a protocol for the pre-selection of fermentation starters. Food Microbiol. 2015, 46, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Chranioti, C.; Kotzekidou, P.; Gerasopoulos, D. Effect of starter cultures on fermentation of naturally and alkali-treated cv. Conservolea green olives. LWT Food Sci. Technol. 2018, 89, 403–408. [Google Scholar] [CrossRef]
- Pino, A.; De Angelis, M.D.; Todaro, A.; Van Hoorde, K.V.; Randazzo, C.L.; Caggia, C. Fermentation of Nocellara Etnea table olives by functional starter cultures at different low salt concentrations. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Gallego, J.; Arroyo-López, F.N.; López-López, A.; Garrido-Fernández, A. Effect of chloride salt mixtures on selected attributes and mineral content of fermented cracked Aloreña olives. LWT Food Sci. Technol. 2011, 44, 120–129. [Google Scholar] [CrossRef]
- Fadda, C.; Del Caro, A.; Sanguinetti, A.M.; Piga, A. Texture and antioxidant evolution of naturally green table olives as affected by different sodium chloride brine concentrations. Grasas Aceites 2014, 65, e002. [Google Scholar]
- WHO. 2012 Guideline: Sodium Intake for Adults and Children; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Rantsiou, K. Salt Reduction in Vegetable Fermentation: Reality or Desire? J. Food Sci. 2013, 78. [Google Scholar]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef]
- Tofalo, R.; Schirone, M.; Perpetuini, G.; Angelozzi, G.; Suzzi, G.; Corsetti, A. Microbiological and chemical profiles of naturally fermented table olives and brines from different Italian cultivars. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2012, 102, 121–131. [Google Scholar] [CrossRef]
- Tataridou, M.; Kotzekidou, P. Fermentation of table olives by oleuropeinolytic starter culture in reduced salt brines and inactivation of Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Uylaşer, V. Changes in phenolic compounds during ripening in Gemlik variety olive fruits obtained from different locations. CYTA J. Food 2015, 13, 167–173. [Google Scholar] [CrossRef] [Green Version]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Rantsiou, K.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Cocolin, L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res. Int. 2013, 50, 135–142. [Google Scholar] [CrossRef]
- Bonatsou, S.; Paramithiotis, S.; Panagou, E.Z. Evolution of yeast consortia during the fermentation of Kalamata natural black olives upon two initial acidification treatments. Front. Microbiol. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, F.; Bautista-Gallego, J.; Arroyo-López, F.N.; Romero-Gil, V.; Jiménez-Díaz, R.; Garrido-Fernández, A.; García-García, P. Tableolive fermentation with multifunctional Lactobacillus pentosus strains. Food Control 2013, 34, 96–105. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C. Changes in volatile compounds and related biochemical profile during controlled fermentation of cv. Conservolea green olives. Food Microbiol. 2006, 23, 738–746. [Google Scholar] [CrossRef]
- Segovia Bravo, K.A.; Arroyo López, F.N.; García García, P.; Durán Quintana, M.C.; Garrido Fernández, A. Treatment of green table olive solutions with ozone. Effect on their polyphenol content and on Lactobacillus pentosus and Saccharomyces cerevisiae growth. Int. J. Food Microbiol. 2007, 114, 60–68. [Google Scholar] [CrossRef]
- Medina, E.; Brenes, M.; García-García, P.; Romero, C.; de Castro, A. Microbial ecology along the processing of Spanish olives darkened by oxidation. Food Control 2018, 86, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Abriouel, H.; Benomar, N.; Cobo, A.; Caballero, N.; Fernández Fuentes, M.Á.; Pérez-Pulido, R.; Gálvez, A. Characterization of lactic acid bacteria from naturally-fermented Manzanilla Aloreña green table olives. Food Microbiol. 2012, 32, 308–316. [Google Scholar] [CrossRef]
- Marsilio, V.; Seghetti, L.; Iannucci, E.; Russi, F.; Lanza, B.; Felicioni, M. Use of a lactic acid bacteria starter culture during green olive (Olea europaea L cv Ascolana tenera) processing. J. Sci. Food Agric. 2005, 85, 1084–1090. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Durante, M.; Ramires, F.A.; Grieco, F.; Tommasi, L.; Perbellini, E.; Falco, V.; Tasioula-Margari, M.; Logrieco, A.F.; Mita, G.; et al. New process for production of fermented black table olives using selected autochthonous microbial resources. Front. Microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.P.; Pereira, J.A.; Bento, A.; Estevinho, M.L. Microbiological characterization of table olives commercialized in Portugal in respect to safety aspects. Food Chem. Toxicol. 2008, 46, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Caruso, G.; Urbani, S.; Schirone, M.; Esposto, S.; Ciarrocchi, A.; Prete, R.; Garcia-Gonzalez, N.; Battistelli, N.; Gucci, R.; et al. Changes in polyphenolic concentrations of table olives (cv. Itrana) produced under different irrigation regimes during spontaneous or inoculated fermentation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Liebeherr, J. Chapter 1: Introduction To Chapter 1: Introduction To. Semant. Web Educ. 2006, 1, 1–15. [Google Scholar]
- Cais-Sokolińska, D. Analysis of metabolic activity of lactic acid bacteria and yeast in model kefirs made from goat’s milk and mixtures of goat’s milk with mare’s milk based on changes in electrical conductivity and impedance. Mljekarstvo 2017, 67, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, D.M.E.; López, A.; Lamarque, A.L. Industrial improvement for naturally black olives production of Manzanilla and Arauco cultivars. J. Food Process. Preserv. 2014, 38, 106–115. [Google Scholar] [CrossRef]
- Rodríguez, H.; Landete, J.M.; Curiel, J.A.; De Las Rivas, B.; Mancheño, J.M.; Muñoz, R. Characterization of the p-coumaric acid decarboxylase from Lactobacillus plantarum CECT 748T. J. Agric. Food Chem. 2008, 56, 3068–3072. [Google Scholar] [CrossRef]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Pistarino, E.; Aliakbarian, B.; Casazza, A.A.; Paini, M.; Cosulich, M.E.; Perego, P. Combined effect of starter culture and temperature on phenolic compounds during fermentation of Taggiasca black olives. Food Chem. 2013, 138, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, C.L.; Fava, G.; Tomaselli, F.; Romeo, F.V.; Pennino, G.; Vitello, E.; Caggia, C. Effect of kaolin and copper based products and of starter cultures on green table olive fermentation. Food Microbiol. 2011, 28, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Laëtitia, G.; Pascal, D.; Yann, D. The Citrate Metabolism in Homo- and Heterofermentative LAB: A Selective Means of Becoming Dominant over Other Microorganisms in Complex Ecosystems. Food Nutr. Sci. 2014, 5, 953–969. [Google Scholar] [CrossRef] [Green Version]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity—A review. S. Afr. J. Enol. Vitic. 2018, 39, 315–329. [Google Scholar] [CrossRef] [Green Version]
- Erasmus, D.J.; Cliff, M.; Van Vuuren, H.J.J. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. Am. J. Enol. Vitic. 2004, 55, 371–378. [Google Scholar]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Potential benefits of the application of yeast starters in table olive processing. Front. Microbiol. 2012, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Luckett, C.R. The Influences of Texture and Mastication Pattern on Flavor Perception Across the Lifespan. Ph.D. Thesis, University of Arkansas, Fayetteville, Arkansas, 2016. [Google Scholar]
- Fernández-Bolaños, J.; Rodríguez, R.; Saldaña, C.; Heredia, A.; Guilén, R.; Jiménez, A. Factors affecting the changes in texture of dressed (“aliñ adas”) olives. Eur. Food Res. Technol. 2002, 214, 237–241. [Google Scholar] [CrossRef]
- Ramírez, E.; Gandul-Rojas, B.; Romero, C.; Brenes, M.; Gallardo-Guerrero, L. Composition of pigments and colour changes in green table olives related to processing type. Food Chem. 2015, 166, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B.; Mínguez-Mosquera, J. Mechanism and Kinetics of the Degradation of Chlorophylls during the Processing of Green Table Olives. J. Agric. Food Chem. 1994, 42, 1089–1095. [Google Scholar] [CrossRef]










| Growth Media | Microorganisms | Method | Incubation Conditions |
|---|---|---|---|
| Plate count agar (PCA) (Merck, Darmstadt, Germany) | Total viable count | Spread plate | 30 °C/72 h |
| De Man-Rogosa-Sharpe agar (MRS) (Oxoid, Basingstoke, UK) + natamycin 0.1% | Lactic acid bacteria | Pour | 30 °C/72 h |
| plate/Overlay | |||
| Sabouraud agar (Oxoid, Basingstoke, UK) | Yeast and Molds | Spread plate | 25 °C/5 d |
| Violet red bile glycose agar (VRBGA) | Enterobacteriacae | Pour | 37 °C/24 h |
| (BD, Sparks, MD) | plate/Overlay | ||
| Violet red bile lactose agar (VRBLA) | Coliforms | Pour | 30 °C/24 h |
| (Oxoid, Basingstoke, UK) | plate/Overlay | ||
| Mannitol salt agar (MSA) (Oxoid, Basingstoke, UK) | Staphylococci | Spread plate | 30 °C/48 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anagnostopoulos, D.A.; Goulas, V.; Xenofontos, E.; Vouras, C.; Nikoloudakis, N.; Tsaltas, D. Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation. Foods 2020, 9, 17. https://doi.org/10.3390/foods9010017
Anagnostopoulos DA, Goulas V, Xenofontos E, Vouras C, Nikoloudakis N, Tsaltas D. Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation. Foods. 2020; 9(1):17. https://doi.org/10.3390/foods9010017
Chicago/Turabian StyleAnagnostopoulos, Dimitrios A., Vlasios Goulas, Eleni Xenofontos, Christos Vouras, Nikolaos Nikoloudakis, and Dimitrios Tsaltas. 2020. "Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation" Foods 9, no. 1: 17. https://doi.org/10.3390/foods9010017
APA StyleAnagnostopoulos, D. A., Goulas, V., Xenofontos, E., Vouras, C., Nikoloudakis, N., & Tsaltas, D. (2020). Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation. Foods, 9(1), 17. https://doi.org/10.3390/foods9010017