Impact of Type and Enzymatic/High Pressure Treatment of Milk on the Quality and Bio-Functional Profile of Yoghurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Treatment and Yoghurt Sample Preparation
- Milk homogenization was performed in a single stage process at 150 bar;
- Thermal treatment of milk was conducted at 95 °C for 5 min;
- TGase (ACTIVA YG, Ajinomoto, DE) was inoculated at an enzyme concentration of 2.0 U·g−1 protein (reference activity: 100 U·g−1);
- The commercial starter culture used was Yo-Mix (Danisco, DK) prepared as a 1:4 (w/w) dilution in commercial UHT (Ultra-High-Temperature) skim milk.
2.2. Fermentation Kinetics
2.3. Study of the Quality Characteristics of Yoghurt
2.4. Study of the Bio-Functional Properties of Yoghurt
2.4.1. Preparation of Water-Soluble Extracts (WSEs)
2.4.2. Determination of ACE-Inhibitory Activity
2.4.3. Determination of Immunomodulatory Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Milk Type and Applied Treatment on the Fermentation Kinetics of Milk
3.2. Effect of Milk Type, Applied Treatment and Storage Time on the Quality Attributes of Yoghurt
3.3. Effect of Milk Type, Applied Treatment and Storage Time on the Anti-Hypertensive Activity of Yoghurt
3.4. Effect of Milk Type, Applied Treatment and Storage Time on the Immunomodulatory Properties of Yoghurt
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teles, C.D.; Flôres, S.H. The influence of additives on the rheological and sensory properties of nonfat yogurt. Int. J. Dairy Technol. 2007, 60, 270–276. [Google Scholar] [CrossRef]
- Tsevdou, M.S.; Eleftheriou, E.G.; Taoukis, P.S. Transglutaminase treatment of thermally and high pressure processed milk: Effects on the properties and storage stability of set yoghurt. Innov. Food Sci. Emerg. 2013, 17, 144–152. [Google Scholar] [CrossRef]
- Bönisch, M.; Huss, M.; Weitl, K.; Kulozik, U. Transglutaminase cross-linking of milk proteins and impact on yoghurt gel properties. Int. Dairy J. 2007, 17, 1360–1371. [Google Scholar] [CrossRef]
- Yüksel, Z.; Erdem, Y.K. The influence of transglutaminase treatment on functional properties of set yoghurt. Int. J. Dairy Technol. 2010, 63, 86–97. [Google Scholar] [CrossRef]
- Şanlı, T.; Sezgin, E.; Deveci, O.; Şenel, E.; Benli, M. Effect of using transglutaminase on physical, chemical and sensory 1 properties of set-type yoghurt. Food Hydrocoll. 2011, 25, 1477–1481. [Google Scholar] [CrossRef]
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Structuring dairy systems through high pressure processing. J. Food Eng. 2013, 114, 106–112. [Google Scholar] [CrossRef]
- Tsevdou, M.; Soukoulis, C.; Cappellin, L.; Gasperi, F.; Taoukis, P.S.; Biasioli, F. Monitoring and Modeling of Endogenous Flavour Compounds Evolution during Fermentation of Thermally, High Hydrostatic Pressure or Transglutaminase Treated Milk using PTR-TOF-MS. Food Chem. 2013, 138, 2159–2167. [Google Scholar] [CrossRef]
- Temiz, H.; Ҫakmak, E. The effect of microbial transglutaminase on probiotic fermented milk produced using a mixture of bovine milk and soy drink. Int. J. Dairy Technol. 2018, 71, 906–920. [Google Scholar] [CrossRef]
- Sakkas, L.; Tsevdou, M.; Zoidou, E.; Gkotzia, E.; Karvounis, A.; Samara, A.; Taoukis, P.; Moatsou, G. Yoghurt-type gels from skim sheep milk base enriched with whey protein concentrate hydrolysates and processed by heating or high hydrostatic pressure. Foods 2019, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, L.; Masotti, F.; Cattaneo, S.; Hogenboom, J.A.; de Noni, I. Nutritional Quality of Milk Proteins. In Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 515–538. [Google Scholar]
- Kohronen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 77–187. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. The scientific evidence for the role of milk protein-derived bioactive peptides in human: A review. J. Funct. Foods 2015, 17, 640–656. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cardiovascular Diseases (CVDs). Available online: www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Silva, S.V.; Malcata, F.X. Caseins as source of bioactive peptides: Review. Int. Dairy J. 2005, 15, 1–15. [Google Scholar] [CrossRef]
- Maruyama, S.; Suzuki, H. A peptide inhibitor of angiotensin I-converting enzyme in the tryptic hydrolysate of casein. Agric. Biol. Chem. 1982, 46, 1393–1394. [Google Scholar]
- Maruyama, S.H.; Mitachi, H.; Awaja, J.; Kurono, M.; Tomizaka, N.; Suzuki, H. Angiotensin I-converting enzyme inhibitor activity of the C-terminal hexapeptide of as1-casein. Agric. Biol. Chem. 1987, 51, 2557–2561. [Google Scholar]
- Maruyama, S.H.; Mitachi, H.; Tanaka, H.; Tomizuka, N.; Suzuki, H. Studies on the active site and antihypertensive activity of angiotensin I-converting enzyme inhibitors derived from casein. Agric. Biol. Chem. 1987, 51, 1581–1586. [Google Scholar] [CrossRef] [Green Version]
- Meisel, H.; Schlimme, E. Milk proteins: Precursors of bioactive peptides. Trends. Food Sci. Technol. 1994, 1, 41–43. [Google Scholar] [CrossRef]
- Politis, I.; Chronopoulou, R. Milk peptides and immune response in the neonate. Adv. Exp. Med. Biol. 2008, 606, 253–269. [Google Scholar]
- Donkor, Ο.Ν.; Henriksson, Α.; Singh, Τ.Κ.; Vasiljevic, Τ.; Shah, N.P. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2004, 17, 1321–1331. [Google Scholar] [CrossRef]
- Politis, I.; Theodorou, G. Angiotensin I-converting (ACE)-inhibitory and anti-inflammatory properties of commercially available Greek yoghurt made from bovine or ovine milk: A comparative study. Int. Dairy J. 2016, 58, 46–49. [Google Scholar] [CrossRef]
- Denkers, E.Y.; Del Rio, L.; Bennouna, S. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem. Immunol. Allergy 2003, 83, 95–114. [Google Scholar]
- Masters, S.L.; Simon, A.; Aksentijevich, I.; Kastner, D.L. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annual Rev. Immunol. 2009, 27, 621–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menter, D.G.; Schilsky, R.L.; DuBois, R.N. Cyclooxygenase-2 and cancer treatment: Understanding the risk should be worth the reward. Clin. Cancer Res. 2010, 16, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, C.H.; So, S.P.; Ruan, K.H. Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: Mechanisms of COX-2 inhibitor risk to heart disease. Life Sci. 2011, 88, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Brabandere, A.G.; de Baerdemaeker, J.G. Effects of process conditions on the pH development during yoghurt fermentation. J. Food Eng. 1999, 41, 221–227. [Google Scholar] [CrossRef]
- Yogurt: Determination of titratable acidity. In International IDF Standard; International Dairy Federation: Brussels, Belgium, 1991.
- Kuchroo, C.N.; Fox, P.F. Soluble nitrogen in Cheddar cheese: Comparison of extraction procedures. Milchwissenschaft 1982, 37, 331–335. [Google Scholar]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Theodorou, G.; Politis, I. Effects of peptides derived from traditional Greek yoghurt on expression of pro- and anti-inflammatory genes by ovine monocytes and neutrophils. Food Agric. Immunol. 2016, 27, 484–495. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Afsar, S.; Day, L. Differences in microstructure and rheological properties of low-fat yoghurts from goat, sheep and cow milk. Food Res. Int. 2018, 108, 423–429. [Google Scholar] [CrossRef]
- Yüksel, Z.; Erdem, Y.K. The influence of main milk components on the hydrophobic interactions of milk protein system in the course of heat treatment. J. Food Eng. 2005, 67, 301–308. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; Upadhyay, V.K.; Kelly, A.L. High-pressure-induced changes in bovine milk: A review. Int. J. Dairy Technol. 2006, 59, 58–66. [Google Scholar] [CrossRef]
- Farnsworth, J.P.; Li, J.; Hendricks, G.M.; Guo, M.R. Effects of transglutaminase treatment on functional properties and probiotic culture survivability of goat milk yogurt. Small Rumin. Res. 2006, 65, 113–121. [Google Scholar] [CrossRef]
- Özer, B.; Kırmacı, H.A.; Oztekin, S.; Hayaloglu, A.; Atamer, M. Incorporation of microbial transglutaminase into non-fat yogurt production. Int. Dairy J. 2007, 17, 199–207. [Google Scholar] [CrossRef]
- Anema, S.G.; Lauber, S.; Lee, S.K.; Henle, T.; Klostermeyer, H. Rheological properties of acid gels prepared from pressure and transglutaminase-treated skim milk. Food Hydrocoll. 2005, 19, 879–887. [Google Scholar] [CrossRef]
- Færgemand, M.; Sørensen, M.V.; Jørgensen, U.; Budolfsen, G.; Qvist, K.B. Transglutaminase: Effect on instrumental and sensory texture of set style yoghurt. Milchwissenschaft 1999, 54, 563–566. [Google Scholar]
- Lauber, S.; Henle, T.; Klostermeyer, H. Relationship between the crosslinking of caseins by transglutaminase and the gel strength of yoghurt. Eur. J. Food Res. Technol. 2000, 210, 305–309. [Google Scholar] [CrossRef]
- Moschopoulou, E.; Sakkas, L.; Zoidou, E.; Theodorou, G.; Sgouridou, E.; Kalathaki, C.; Liarakou, A.; Chatzigeorgiou, A.; Politis, I.; Moatsou, G. Effect of milk kind and storage on the biochemical, textural and biofunctional characteristics of set-type yoghurt. Int. Dairy J. 2018, 77, 47–55. [Google Scholar] [CrossRef]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef]
- Cermẽno, M.; FitzGerald, R.J.; O’Brien, N.M. In vitro antioxidant and immunomodulatory activity of transglutaminase-treated sodium caseinate hydrolysates. Int. Dairy J. 2016, 63, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-beta. Annual Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [Green Version]
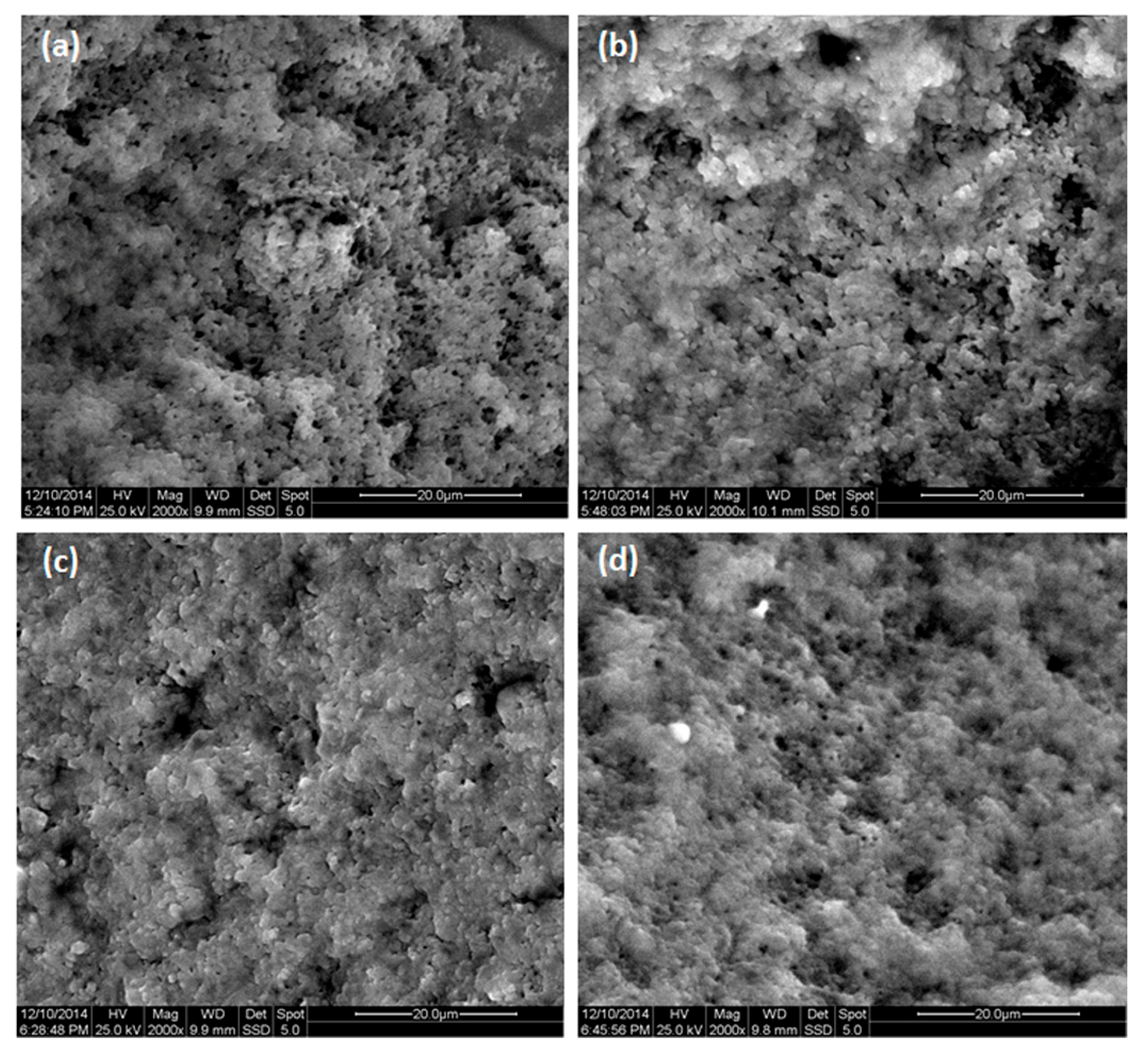
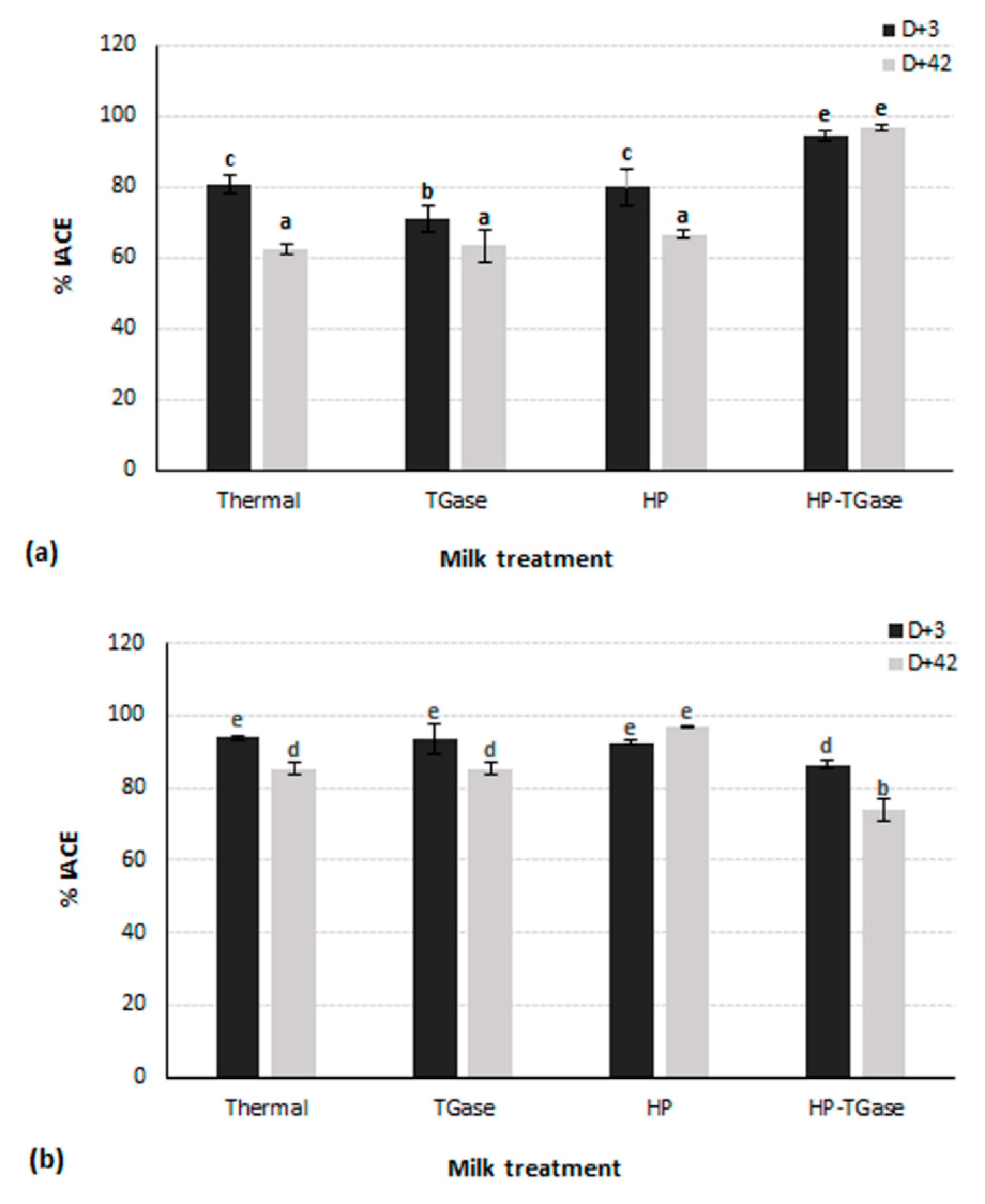
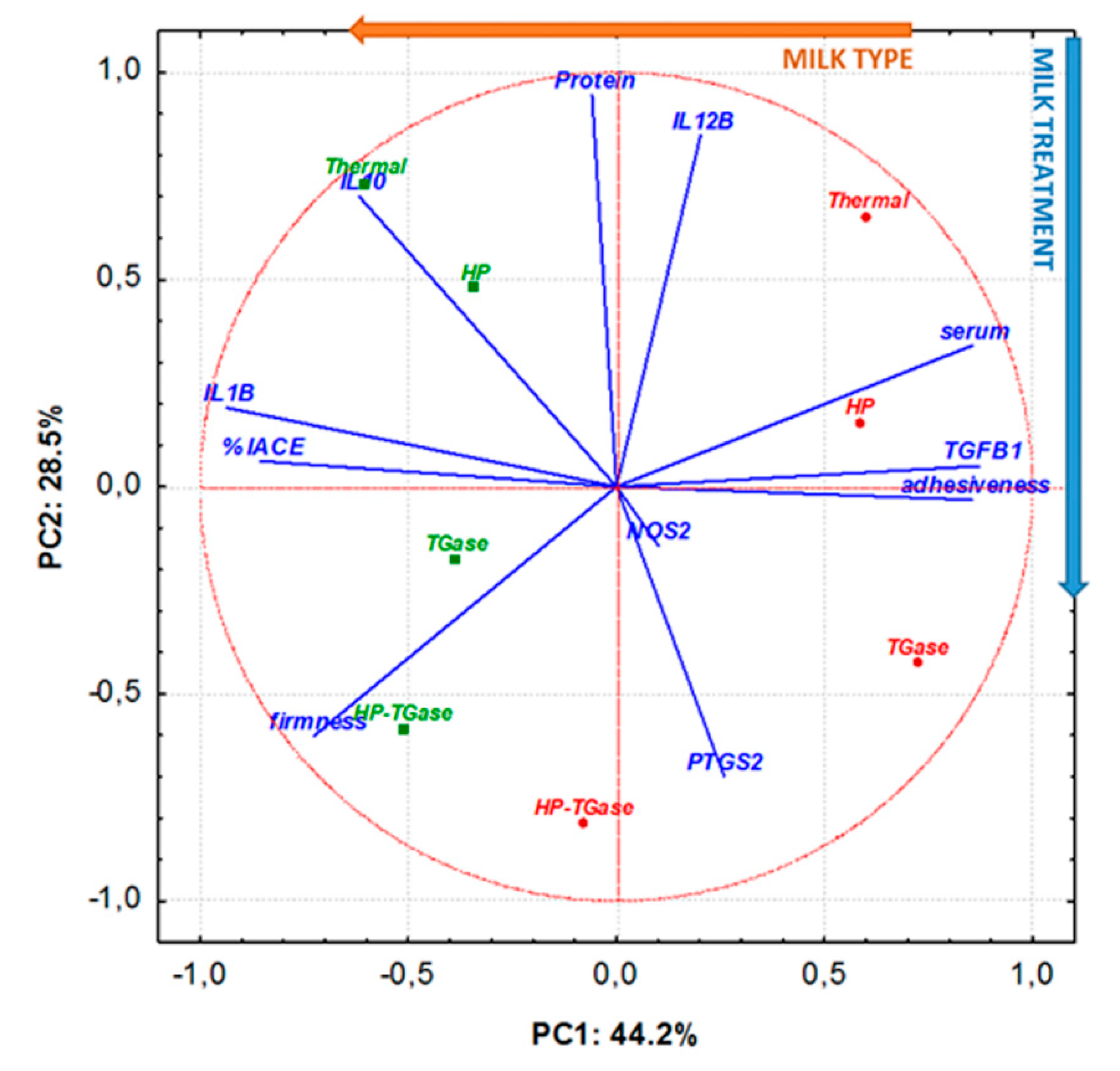
| Code | Type of Milk | Performed Treatment for Milk Sample |
|---|---|---|
| Thermal | Bovine or Ovine | Thermal treatment at 95 °C for 5 min |
| TGase | Thermal treatment at 95 °C for 5 min, followed by TGase treatment at 43 °C for 180 min and inactivation of the enzyme at 80 °C for 1 min | |
| HP | HP treatment at 600 MPa and 55 °C for 10 min | |
| HP–TGase | HP treatment at 600 MPa and 55 °C for 10 min, followed by TGase treatment at 43 °C for 180 min and inactivation of the enzyme at 80 °C for 1 min |
| Lag Phase λ (min) | Maximum Acidification Rate μ (pH/min) | R2 | Fermentation Time (min) | |
|---|---|---|---|---|
| Bovine | ||||
| Thermal | 116 f (± 1.1) | 0.0181 b (± 0.0004) | 0.999 | 220 bc (± 15) |
| TGase | 90.4 b (± 0.6) | 0.0190 cd (± 0.0012) | 0.998 | 190 a (± 10) |
| HP | 123 g (± 0.0) | 0.0198 d (± 0.0000) | 0.996 | 230 cd (± 0.0) |
| HP–TGase | 107 e (± 0.0) | 0.0185 bc (± 0.0000) | 0.998 | 210 b (± 5.0) |
| Ovine | ||||
| Thermal | 93.2 c (± 1.2) | 0.0151 a (± 0.0002) | 0.992 | 260 e (± 5.0) |
| TGase | 93.3 c (± 0.1) | 0.0147 a (± 0.0001) | 0.995 | 240 d (± 0.0) |
| HP | 103 d (± 0.2) | 0.0196 cd (± 0.0001) | 0.997 | 210 b (± 0.0) |
| HP–TGase | 88.1a (± 1.0) | 0.0178 b (± 0.0001) | 0.998 | 210 b (± 5.0) |
| Physicochemical Characteristics | Main Textural Attributes | |||||
|---|---|---|---|---|---|---|
| pH | % Lactic Acid | Serum (g/100g Product) | Firmness (g) | Adhesiveness (g·s) | Cohesiveness * | |
| Bovine | ||||||
| Thermal | 4.48 e (± 0.01) | 0.79 b (± 0.01) | 49.8 f (± 1.7) | 74.3 a (± 3.8) | 38.1 ef (± 1.0) | 0.52 b (± 0.01) |
| TGase | 4.44 d (± 0.02) | 0.79 b (± 0.01) | 39.6 e (± 1.4) | 146 b (± 2.5) | 34.3 f (± 2.7) | 0.53 bc (± 0.01) |
| HP | 4.51 ef (± 0.04) | 0.98 e (± 0.00) | 47.5 f (± 0.6) | 89.0 a (± 6.0) | 44.6 e (± 4.7) | 0.52 b (± 0.02) |
| HP–TGase | 4.18 a (± 0.01) | 0.96 e (± 0.02) | 32.9 d (± 2.9) | 333 d (± 6.7) | 121 b (± 8.9) | 0.44 a (± 0.01) |
| Ovine | ||||||
| Thermal | 4.42 cd (± 0.02) | 0.82 bc (± 0.03) | 23.8 c (± 0.8) | 220 c (± 6.8) | 152 a (± 3.7) | 0.51 b (± 0.02) |
| TGase | 4.54 f (± 0.02) | 0.72 a (± 0.01) | 13.8 b (± 1.0) | 338 d (± 14) | 80.1 d (± 0.4) | 0.60 d (± 0.01) |
| HP | 4.40 bc (± 0.01) | 0.86 cd (± 0.02) | 32.2 d (± 1.1) | 219 c (± 2.8) | 96.1 c (± 2.3) | 0.55 c (± 0.00) |
| HP–TGase | 4.37 b (± 0.02) | 0.88d (± 0.05) | 8.76 a (± 0.9) | 548 e (± 18) | 94.3 c (± 0.2) | 0.62 d (± 0.01) |
| IL1B | IL10 | IL12B | PTGS2 | NOS2 | TGFB1 | |
|---|---|---|---|---|---|---|
| Bovine | ||||||
| D+3 | ||||||
| Thermal | 0.952 abc (± 0.027) | 2.428 b (± 0.051) | 0.562 a (± 0.066) | 1.690 ab (± 0.033) | 4.150 cd (± 0.583) | 2.133 def (± 0.226) |
| TGase | 0.972 abc (± 0.090) | 1.460 a (± 0.339) | 0.312 a (± 0.051) | 1.884 ab (± 0.341) | 3.106 ab (± 0.907) | 2.291 ef (± 0.150) |
| HP | 0.815 ab (± 0.072) | 1.441 a (± 0.213) | 0.501 a (± 0.143) | 1.430 a (± 0.146) | 2.862a (± 0.088) | 1.900 bcde (± 0.209) |
| HP–TGase | 1.159 c (± 0.074) | 1.427 a (± 0.216) | 0.366 a (± 0.089) | 2.481 bc (± 0.112) | 3.139 ab (± 0.743) | 1.574 abc (± 0.286) |
| D+42 | ||||||
| Thermal | 0.772 a (± 0.088) | 1.446 a (± 0.093) | 0.478 a (± 0.132) | 1.330 a (± 0.093) | 2.724 a (± 0.436) | 1.621 abc (± 0.141) |
| TGase | 1.096 abc (± 0.176) | 1.359 a (± 0.151) | 0.318 a (± 0.019) | 1.421 a (± 0.142) | 3.210 ab (± 0.294) | 1.735 abcd (± 0.236) |
| HP | 1.046 abc (± 0.064) | 1.495 a (± 0.118) | 0.521 a (± 0.121) | 1.360 a (± 0.051) | 2.995 a (± 0.144) | 1.436 a (± 0.442) |
| HP–TGase | 1.111 bc (± 0.064) | 1.242 a (± 0.181) | 0.319 a (± 0.080) | 1.637 ab (± 0.256) | 4.093 cd (± 0.643) | 1.545 ab (± 0.081) |
| Ovine | ||||||
| D+3 | ||||||
| Thermal | 2.319 f (± 0.217) | 3.052 c (± 0.326) | 2.366 c (± 0.032) | 3.073 cd (± 0.353) | 5.508 efg (± 0.638) | 2.448 fg (± 0.058) |
| TGase | 1.896 e (± 0.228) | 2.404 b (± 0.463) | 1.640 b (± 0.043) | 3.358 d (± 0.559) | 5.272 ef (± 0.440) | 1.978 cde (± 0.009) |
| HP | 2.109 ef (± 0.238) | 2.491 b (± 0.536) | 3.253 d (± 0.235) | 4.964 e (± 0.387) | 3.448 abc (± 0.378) | 3.058 h (± 0.279) |
| HP–TGase | 2.130 ef (± 0.324) | 2.132 b (± 0.194) | 1.888 b (± 0.095) | 3.503 d (± 0.590) | 5.451 efg (± 0.358) | 2.412 f (± 0.382) |
| D+42 | ||||||
| Thermal | 3.085 g (± 0.259) | 3.176c (± 0.366) | 5.912 e (± 0.564) | 4.754 e (± 0.180) | 6.182 g (± 0.100) | 3.820 j (± 0.114) |
| TGase | 1.481 d (± 0.251) | 1.409 a (± 0.028) | 2.480 c (± 0.291) | 2.111 ab (± 0.098) | 4.708 de (± 0.009) | 1.668 abc (± 0.033) |
| HP | 2.123 ef (± 0.176) | 2.512 b (± 0.106) | 3.489 d (± 0.336) | 3.588 d (± 0.640) | 5.785 fg (± 0.292) | 2.515 fg (± 0.198) |
| HP–TGase | 2.057 ef (± 0.078) | 3.231 c (± 0.140) | 5.979 e (± 0.556) | 4.455 e (± 0.104) | 3.922 bcd (± 0.302) | 2.815 gh (± 0.163) |
| Milk Type (MT) | *** | *** | *** | *** | *** | *** |
| Milk Treatment (MT) | *** | *** | *** | *** | *** | *** |
| Storage Time (ST) | n.s. | n.s. | *** | n.s. | n.s. | n.s. |
| MO × MT × ST | *** | *** | *** | *** | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsevdou, M.; Theodorou, G.; Pantelaiou, S.; Chatzigeorgiou, A.; Politis, I.; Taoukis, P. Impact of Type and Enzymatic/High Pressure Treatment of Milk on the Quality and Bio-Functional Profile of Yoghurt. Foods 2020, 9, 49. https://doi.org/10.3390/foods9010049
Tsevdou M, Theodorou G, Pantelaiou S, Chatzigeorgiou A, Politis I, Taoukis P. Impact of Type and Enzymatic/High Pressure Treatment of Milk on the Quality and Bio-Functional Profile of Yoghurt. Foods. 2020; 9(1):49. https://doi.org/10.3390/foods9010049
Chicago/Turabian StyleTsevdou, Maria, Georgios Theodorou, Sofia Pantelaiou, Artemis Chatzigeorgiou, Ioannis Politis, and Petros Taoukis. 2020. "Impact of Type and Enzymatic/High Pressure Treatment of Milk on the Quality and Bio-Functional Profile of Yoghurt" Foods 9, no. 1: 49. https://doi.org/10.3390/foods9010049
APA StyleTsevdou, M., Theodorou, G., Pantelaiou, S., Chatzigeorgiou, A., Politis, I., & Taoukis, P. (2020). Impact of Type and Enzymatic/High Pressure Treatment of Milk on the Quality and Bio-Functional Profile of Yoghurt. Foods, 9(1), 49. https://doi.org/10.3390/foods9010049







