Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis of WPC
2.3. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Response Surface Methodology (RSM) Design for WPC Hydrolysis
2.5. Degree of Hydrolysis (DH)
2.6. Angiotensin Converting Enzyme (Ace) Inhibitory Activity Assay
2.7. Determination of Antioxidant Activity
2.7.1. 1, 1-diphenyl-2-picrylhydrazyl Free Radical Scavenging Assay
2.7.2. Ferrous Ion Chelating Activity Assay
3. Results and Discussion
3.1. Proximate Analysis
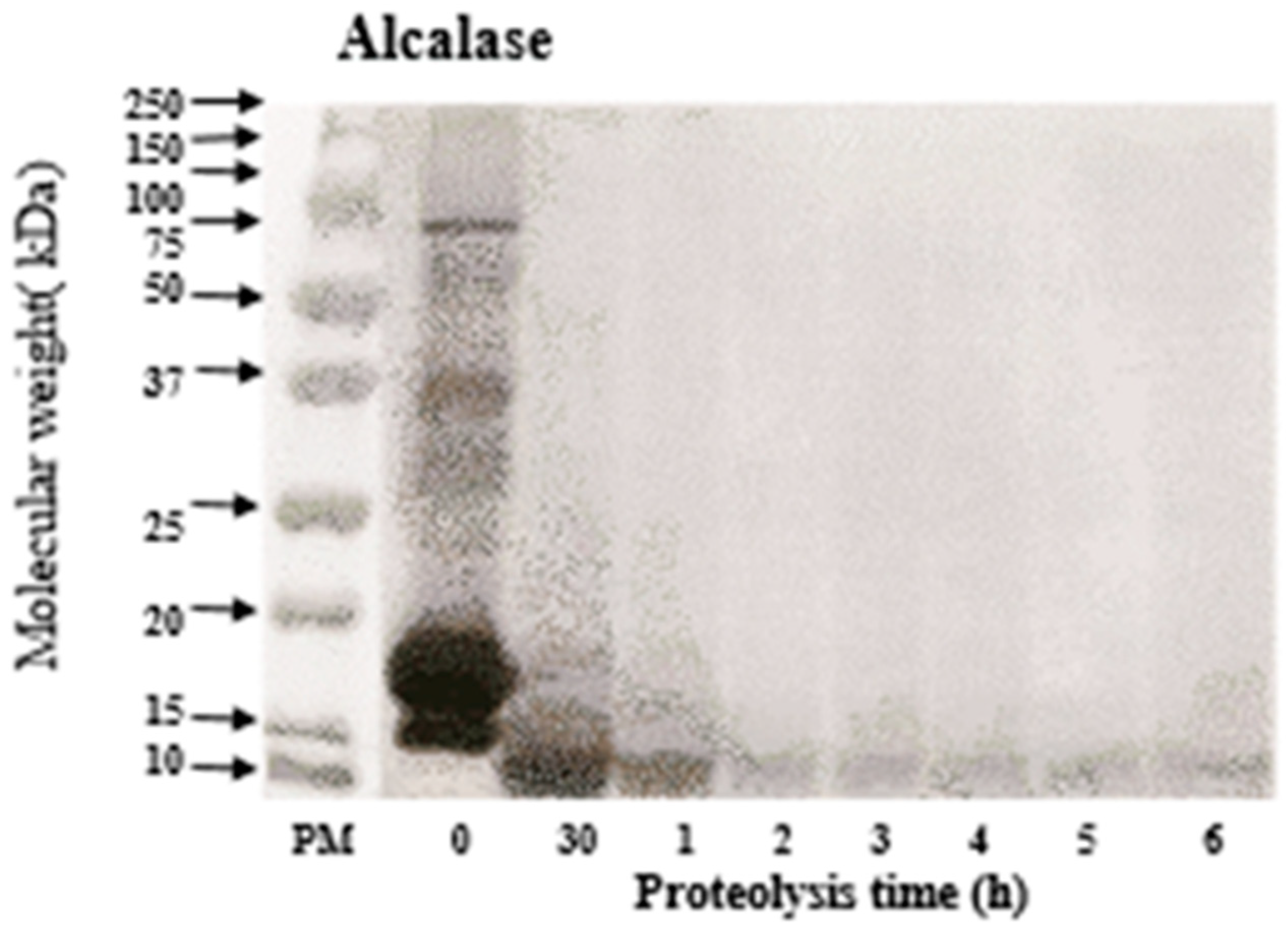
3.2. Molecular Weight of WPC Hydrolysates
3.3. Model Performance Appraisal on the Regression Elements
3.4. Effects of Temperature, E/S Ratio, pH and Time on Different Responses
3.4.1. Degree of Hydrolysis (DH)
3.4.2. ACE-inhibitory Activity
3.4.3. Antioxidant Activities
3.5. Optimized Hydrolysis Condition For WPC and Model Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19 1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Adamson, N. Whey processing. In Dairy Processing Handbook; Bylund, G., Ed.; Tetra Pak Processing Systems: Lund, Sweden, 2015; pp. 331–352. [Google Scholar]
- Suthar, J.; Jana, A.; Balakrishnan, S. High protein milk ingredients-A tool for value-addition to dairy and food products. J. Dairy Vet. Anim. Res. 2017, 6, 00171. [Google Scholar]
- Whetstine, M.C.; Croissant, A.; Drake, M. Characterization of dried whey protein concentrate and isolate flavor. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Hernández-Ledesma, B.; Amigo, L.; Martín-Álvarez, P.J.; Recio, I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology. LWT Food Sci. Technol. 2011, 44, 9–15. [Google Scholar] [CrossRef]
- Muro Urista, C.; Álvarez Fernández, R.; Riera Rodriguez, F.; Arana Cuenca, A.; Tellez Jurado, A. Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Tian, W.; Li, H.; Cao, J.; Jiang, W. Improving antioxidant activities of whey protein hydrolysates obtained by thermal preheat treatment of pepsin, trypsin, alcalase and flavourzyme. Int. J. Food Sci. Technol. 2012, 47, 2045–2051. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Tanokura, M. Optimisation of hydrolysis conditions for the production of the angiotensin-I converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology. Food Chem. 2009, 114, 328–333. [Google Scholar] [CrossRef]
- Morais, H.A.; Silvestre, M.P.C.; Silva, M.R.; Silva, V.D.M.; Batista, M.A.; e Silva, A.C.S.; Silveira, J.N. Enzymatic hydrolysis of whey protein concentrate: Effect of enzyme type and enzyme: Substrate ratio on peptide profile. J. Food Sci. Technol. 2015, 52, 201–210. [Google Scholar] [CrossRef]
- Tavares, T.; Malcata, F. Whey proteins as source of bioactive peptides against hypertension. In Bioactive Food Peptides in Health and Disease; Hernández-Ledesma, B., Ed.; InTech Publisher: London, UK, 2013; pp. 75–114. [Google Scholar]
- Wu, Q.; Zhang, X.; Jia, J.; Kuang, C.; Yang, H. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: Thermodynamic parameters, physicochemical properties and bioactivities. Process Biochem. 2018, 67, 46–54. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez Maqueda, D.; Recio, I.; Hernández Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Boyacı, D.; Korel, F.; Yemenicioğlu, A. Development of activate-at-home-type edible antimicrobial films: An example pH-triggering mechanism formed for smoked salmon slices using lysozyme in whey protein films. Food Hydrocoll. 2016, 60, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Madureira, A.; Tavares, T.; Gomes, A.M.P.; Pintado, M.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’keeffe, M.B.; Conesa, C.; FitzGerald, R.J. Identification of angiotensin converting enzyme inhibitory and antioxidant peptides in a whey protein concentrate hydrolysate produced at semi-pilot scale. Int. J. Food Sci. Technol. 2017, 52, 1751–1759. [Google Scholar] [CrossRef]
- Ajlouni, S.; Pan, Y. Effect of pH and whey protein isolate to glucose ratios on the formation of Maillard reaction products as antioxidants. Kasetsart J. (Nat. Sci.) 2014, 48, 759–768. [Google Scholar]
- Zhang, Q.X.; Wu, H.; Ling, Y.F.; Lu, R.R. Isolation and identification of antioxidant peptides derived from whey protein enzymatic hydrolysate by consecutive chromatography and Q-TOF MS. J. Dairy Res. 2013, 80, 367–373. [Google Scholar] [CrossRef]
- Naik, L.; Mann, B.; Bajaj, R.; Sangwan, R.; Sharma, R. Process optimization for the production of bio-functional whey protein hydrolysates: Adopting response surface methodology. Int. J. Pept. Res. 2013, 19, 231–237. [Google Scholar] [CrossRef]
- Athira, S.; Mann, B.; Saini, P.; Sharma, R.; Kumar, R.; Singh, A.K. Production and characterisation of whey protein hydrolysate having antioxidant activity from cheese whey. J. Sci. Food Agric. 2015, 95, 2908–2915. [Google Scholar] [CrossRef]
- Fenoglio, C.; Vierling, N.; Manzo, R.; Ceruti, R.; Sihufe, G.; Mammarella, E. Whey protein hydrolysis with free and immobilized alcalase®: Effects of operating parameters on the modulation of peptide profiles obtained. Am. J. Food Technol. 2016, 11, 152–158. [Google Scholar]
- Bezerra, M.; Santelli, R.; Oliveira, E.; Villar, L.; Escaleira, L. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists (AOAC) International: Rockville, MD, USA, 2005. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, E. Introduction to multivariate regression analysis. Hippokratia 2010, 14, 23–28. [Google Scholar] [PubMed]
- Mayer, R.; Montgomery, D. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley and Sons, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Church, F.; Swaisgood, H.; Porter, D.; Catignani, G. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Salami, M.; Yousefi, R.; Ehsani, M.R.; Dalgalarrondo, M.; Chobert, J.M.; Haertlé, T.; Razavi, S.H.; Saboury, A.A.; Niasari-Naslaji, A.; Moosavi-Movahedi, A.A. Kinetic characterization of hydrolysis of camel and bovine milk proteins by pancreatic enzymes. Int. Dairy J. 2008, 18, 1097–1102. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- Cushman, D.; Cheung, H. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharm. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Mota, M.; Tavares, P.; Pereira, A.; Goncalves, M.; Torres, D.; Rocha, C.; Teixeira, J. Preparation of ingredients containing an ACE-inhibitory peptide by tryptic hydrolysis of whey protein concentrates. Int. Dairy J. 2007, 17, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.Y.; Shyu, Y.S.; Wang, Y.T.; Hsu, C.K. Antioxidative properties of protein hydrolysate from defatted peanut kernels treated with esperase. LWT Food Sci. Technol. 2010, 43, 285–290. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Pena-Ramos, E.; Xiong, Y. Antioxidative activity of whey protein hydrolysates in a liposomal system. J. Dairy Sci. 2001, 84, 2577–2583. [Google Scholar] [CrossRef]
- See, S.F.; Hoo, L.L.; Babji, A.S. Optimization of enzymatic hydrolysis of Salmon (Salmo salar) skin by Alcalase. Int. Food Res. J. 2011, 18, 1359–1365. [Google Scholar]
- Silvestre, M.P.C.; Silva, M.R.; Silva, V.D.M.; Souza, M.W.S.; Junior, L.; Oliveira, C.; Afonso, W.O. Analysis of whey protein hydrolysates: Peptide profile and ACE inhibitory activity. Braz. J. Pharm. Sci. 2012, 48, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I converting enzyme (ACE) inhibitory and anti-oxidant activities of sea cucumber (Actinopyga lecanora) hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Ven, C.; Gruppen, H.; de Bont, D.; Voragen, A. Optimisation of the angiotensin converting enzyme inhibition by whey protein hydrolysates using response surface methodology. Int. Dairy J. 2002, 12, 813–820. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [Green Version]
- O’Loughlin, I.B.; Kelly, P.M.; Murray, B.A.; FitzGerald, R.J.; Brodkorb, A. Molecular characterization of whey protein hydrolysate fractions with ferrous chelating and enhanced iron solubility capabilities. J. Agric. Food Chem. 2015, 63, 2708–2714. [Google Scholar] [CrossRef]







| Parameters | Present Study (Mean ± sd) | Literature Data * |
|---|---|---|
| Moisture (%) | 5.64 ± 0.03 | 3.8–4.5 |
| Protein (%) | 76.13 ± 0.23 | 74.8–80.0 |
| Crude fat (%) | 3.91 ± 0.75 | 1.8–7.2 |
| Ash (%) | 2.96 ± 0.01 | 2.7–7.5 |
| Carbohydrates (%) | 11.93 ± 0.00 | 3.5–8.9 |
| Run Order | x1 | x2 | x3 | x4 | % DH (y1) | %ACE Inhibition (y2) | % DPPH• (y3) | % Ferrous Ion Chelating (y4) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental | Predicted | |||||
| 1 | 40 | 0.5 | 8.25 | 255 | 30.71 | 45.22 | 27.53 | 26.85 | 27.53 | 26.85 | 73.78 | 72.09 |
| 2 | 70 | 0.5 | 8.25 | 255 | 44.63 | 52.36 | 51.10 | 46.28 | 51.10 | 46.28 | 76.30 | 74.33 |
| 3 | 40 | 2.5 | 8.25 | 255 | 67.42 | 63.67 | 20.97 | 26.23 | 20.97 | 26.23 | 65.04 | 69.37 |
| 4 | 70 | 2.5 | 8.25 | 255 | 62.09 | 70.81 | 47.13 | 45.65 | 47.13 | 45.65 | 74.70 | 71.61 |
| 5 | 55 | 1.5 | 6.5 | 30 | 22.67 | 25.22 | 31.87 | 35.59 | 31.87 | 35.59 | 73.48 | 68.97 |
| 6 | 55 | 1.5 | 10 | 30 | 42.64 | 43.29 | 51.47 | 54.03 | 51.47 | 54.03 | 58.00 | 62.23 |
| 7 | 55 | 1.5 | 6.5 | 480 | 87.35 | 77.50 | 49.10 | 55.03 | 49.10 | 55.03 | 65.63 | 64.82 |
| 8 | 55 | 1.5 | 10 | 480 | 91.07 | 95.57 | 40.77 | 45.53 | 40.77 | 45.53 | 58.37 | 58.08 |
| 9 | 40 | 1.5 | 8.25 | 30 | 17.90 | 18.09 | 34.20 | 23.81 | 34.20 | 23.81 | 63.44 | 63.51 |
| 10 | 70 | 1.5 | 8.25 | 30 | 34.46 | 25.23 | 41.33 | 43.23 | 41.33 | 43.23 | 64.04 | 65.75 |
| 11 | 40 | 1.5 | 8.25 | 480 | 72.37 | 70.37 | 21.67 | 29.27 | 21.67 | 29.27 | 60.93 | 59.36 |
| 12 | 70 | 1.5 | 8.25 | 480 | 66.52 | 77.52 | 51.77 | 48.7 | 51.77 | 48.7 | 59.00 | 61.60 |
| 13 | 55 | 0.5 | 6.5 | 255 | 40.58 | 42.54 | 42.80 | 45.62 | 42.8 | 45.62 | 77.00 | 77.55 |
| 14 | 55 | 2.5 | 6.5 | 255 | 79.65 | 80.60 | 57.73 | 45.00 | 57.73 | 45.00 | 74.96 | 74.83 |
| 15 | 55 | 0.5 | 10 | 255 | 82.06 | 80.22 | 48.33 | 50.09 | 48.33 | 50.09 | 69.96 | 70.81 |
| 16 | 55 | 2.5 | 10 | 255 | 81.91 | 79.06 | 42.93 | 49.46 | 42.93 | 49.46 | 67.59 | 68.09 |
| 17 | 40 | 1.5 | 6.5 | 255 | 58.15 | 54.87 | 24.5 | 24.31 | 24.5 | 24.31 | 66.56 | 71.16 |
| 18 | 70 | 1.5 | 6.5 | 255 | 51.01 | 43.09 | 32.8 | 43.73 | 32.8 | 43.73 | 73.11 | 73.40 |
| 19 | 40 | 1.5 | 10 | 255 | 59.72 | 54.02 | 30.4 | 28.77 | 30.4 | 28.77 | 70.19 | 64.42 |
| 20 | 70 | 1.5 | 10 | 255 | 90.43 | 80.08 | 51.70 | 48.20 | 51.70 | 48.20 | 66.22 | 66.66 |
| 21 | 55 | 0.5 | 8.25 | 30 | 27.44 | 25.03 | 42.37 | 45.12 | 42.37 | 45.12 | 73.22 | 73.43 |
| 22 | 55 | 2.5 | 8.25 | 30 | 35.25 | 43.48 | 44.57 | 44.50 | 44.57 | 44.50 | 65.37 | 63.64 |
| 23 | 55 | 0.5 | 8.25 | 480 | 83.48 | 77.31 | 60.13 | 50.59 | 60.13 | 50.59 | 61.22 | 62.21 |
| 24 | 55 | 2.5 | 8.25 | 480 | 93.28 | 95.76 | 55.17 | 49.96 | 55.17 | 49.96 | 67.51 | 66.56 |
| 25 | 55 | 1.5 | 8.25 | 255 | 60.21 | 70.61 | 48.17 | 47.54 | 48.17 | 47.54 | 75.93 | 75.76 |
| 26 | 55 | 1.5 | 8.25 | 255 | 76.95 | 70.61 | 49.53 | 47.54 | 49.53 | 47.54 | 74.93 | 75.76 |
| 27 | 55 | 1.5 | 8.25 | 255 | 72.94 | 70.61 | 48.30 | 47.54 | 48.30 | 47.54 | 75.41 | 75.76 |
| Factors | y1 | p-Value | y2 | p-Value | y3 | p-Value | y4 | p-Value |
|---|---|---|---|---|---|---|---|---|
| Regression | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Linear | 0.000 | 0.004 | 0.000 | 0.000 | ||||
| Squares | 0.001 | 0.000 | 0.000 | 0.000 | ||||
| Interaction | 0.013 | 0.000 | 0.038 | 0.024 | ||||
| Constant | −116.761 | 0.203 | −57.8367 | 0.408 | −190.337 | 0.000 | −31.8992 | 0.355 |
| x1 | 3.421 | 0.110 | −0.9430 | 0.428 | 6.167 | 0.000 | 1.9866 | 0.003 |
| x2 | 55.453 | 0.010 | 33.1444 | 0.007 | −0.313 | 0.865 | −5.3641 | 0.009 |
| x3 | −6.253 | 0.511 | 34.2940 | 0.007 | 5.799 | 0.020 | 13.9083 | 0.041 |
| x4 | 0.219 | 0.000 | 0.0542 | 0.210 | 0.158 | 0.027 | 0.0609 | 0.001 |
| x1*x1 | −0.056 | 0.001 | −0.0286 | 0.003 | −0.050 | 0.000 | −0.0174 | 0.004 |
| x2*x2 | NS | NS | NS | NS | ||||
| x3*x3 | NS | −3.3472 | 0.000 | NS | −0.9596 | 0.022 | ||
| x4*x4 | 0.000 | 0.005 | −0.0002 | 0.000 | NS | −0.0002 | 0.000 | |
| x1*x2 | NS | NS | NS | NS | ||||
| x1*x3 | 0.360 | 0.032 | 0.4761 | 0.000 | NS | NS | ||
| x1*x4 | NS | 0.0014 | 0.050 | NS | NS | |||
| x2*x3 | −5.603 | 0.027 | −4.0816 | 0.006 | NS | NS | ||
| x2*x4 | NS | NS | NS | 0.0157 | 0.024 | |||
| x3*x4 | NS | NS | −0.018 | 0.038 | NS | |||
| R2 | 91.27% | 89.96% | 74.62% | 84.56% | ||||
| R2-adj | 87.40% | 83.68% | 67.01% | 77.70% | ||||
| Lack of fit | 0.665 | 0.060 | 0.013 | 0.027 | ||||
| f-value | 23.54 | 14.33 | 9.80 | 12.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, F.A.; Chay, S.Y.; Zarei, M.; Auwal, S.M.; Hamid, A.A.; Wan Ibadullah, W.Z.; Saari, N. Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study. Foods 2020, 9, 64. https://doi.org/10.3390/foods9010064
Hussein FA, Chay SY, Zarei M, Auwal SM, Hamid AA, Wan Ibadullah WZ, Saari N. Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study. Foods. 2020; 9(1):64. https://doi.org/10.3390/foods9010064
Chicago/Turabian StyleHussein, Fatima Abdelhameed, Shyan Yea Chay, Mohammad Zarei, Shehu Muhammad Auwal, Azizah Abdul Hamid, Wan Zunairah Wan Ibadullah, and Nazamid Saari. 2020. "Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study" Foods 9, no. 1: 64. https://doi.org/10.3390/foods9010064
APA StyleHussein, F. A., Chay, S. Y., Zarei, M., Auwal, S. M., Hamid, A. A., Wan Ibadullah, W. Z., & Saari, N. (2020). Whey Protein Concentrate as a Novel Source of Bifunctional Peptides with Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Properties: RSM Study. Foods, 9(1), 64. https://doi.org/10.3390/foods9010064






