Addition of Coriander during Fermentation of Korean Soy Sauce (Gangjang) Causes Significant Shift in Microbial Composition and Reduction in Biogenic Amine Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Soy Sauce Samples
2.2. DNA Extraction, Sequencing, and Metagenomic Analysis
2.3. Quantification of Biogenic Amines Using 1H NMR Spectroscopy
2.4. Statistical Analysis
3. Results
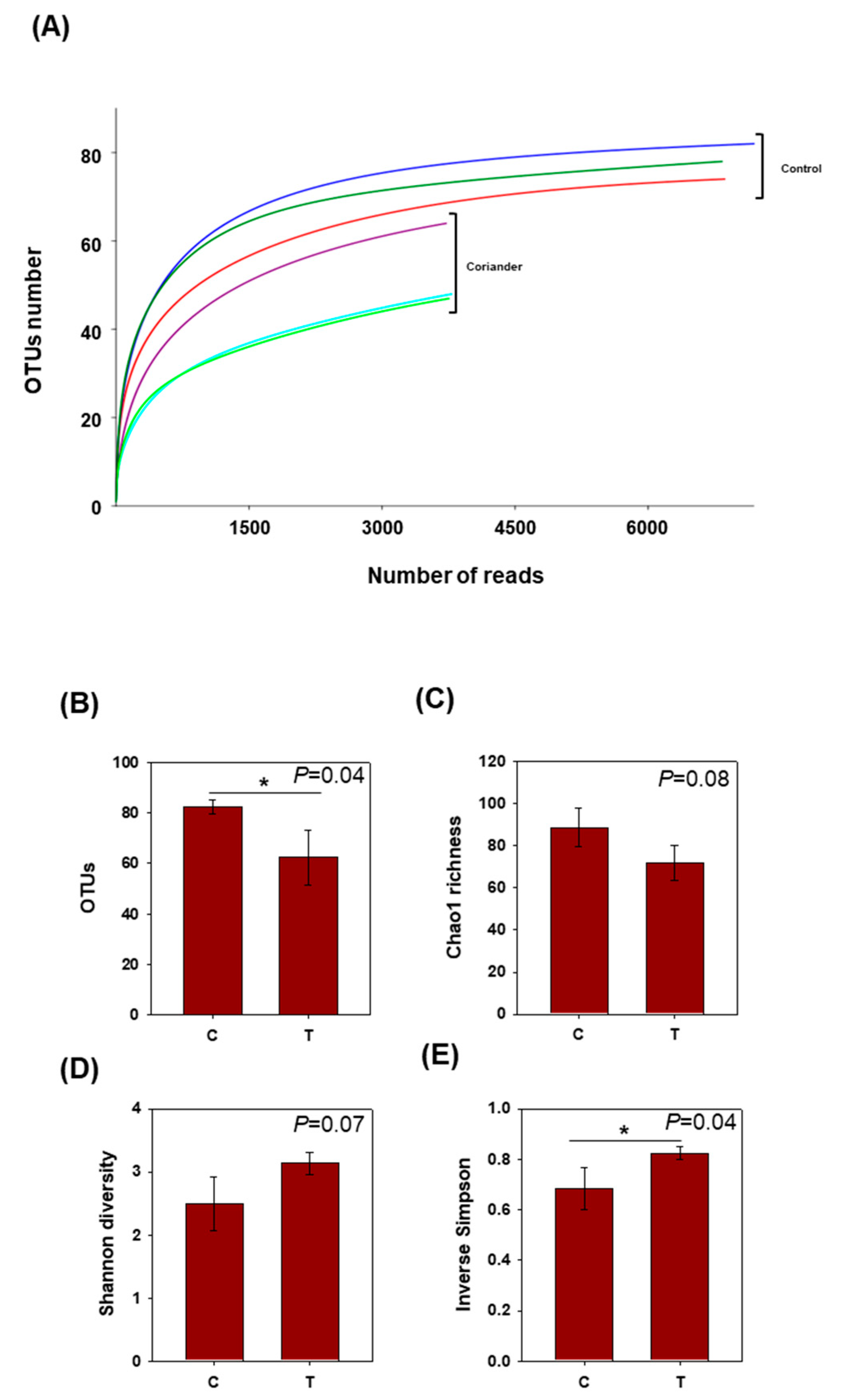
3.1. Microbial Diversity in Soy Sauce Samples Prepared with or without Coriander
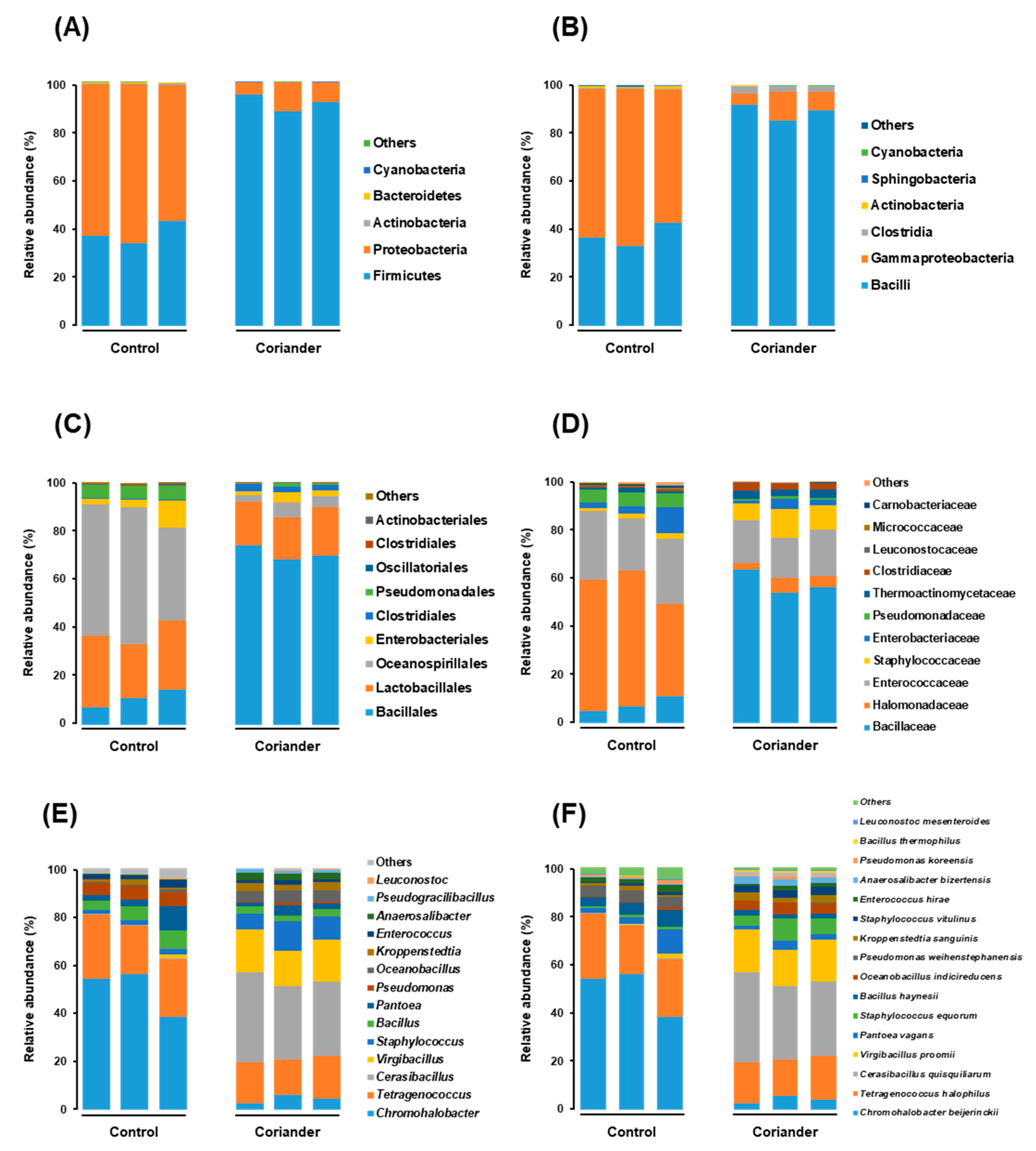
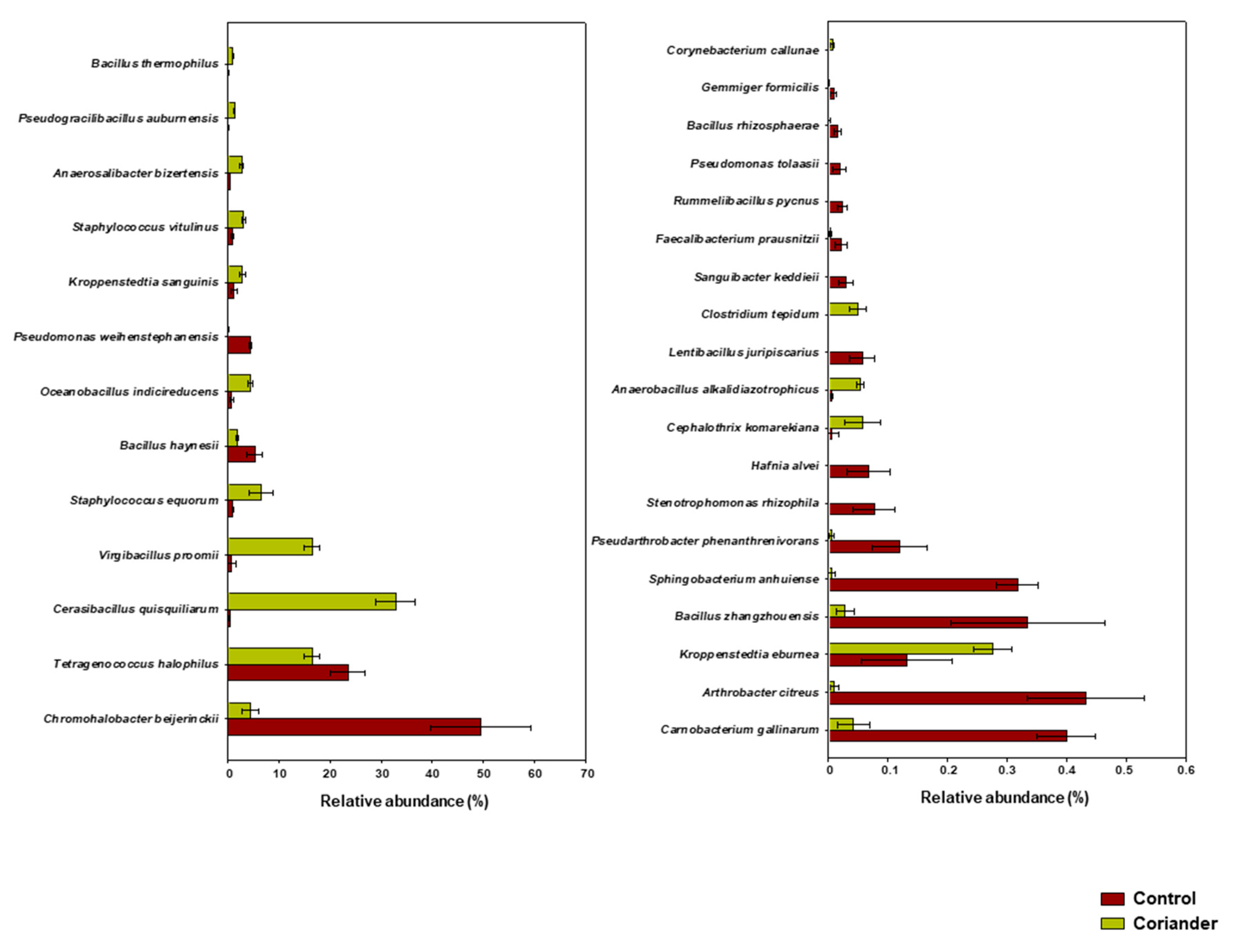
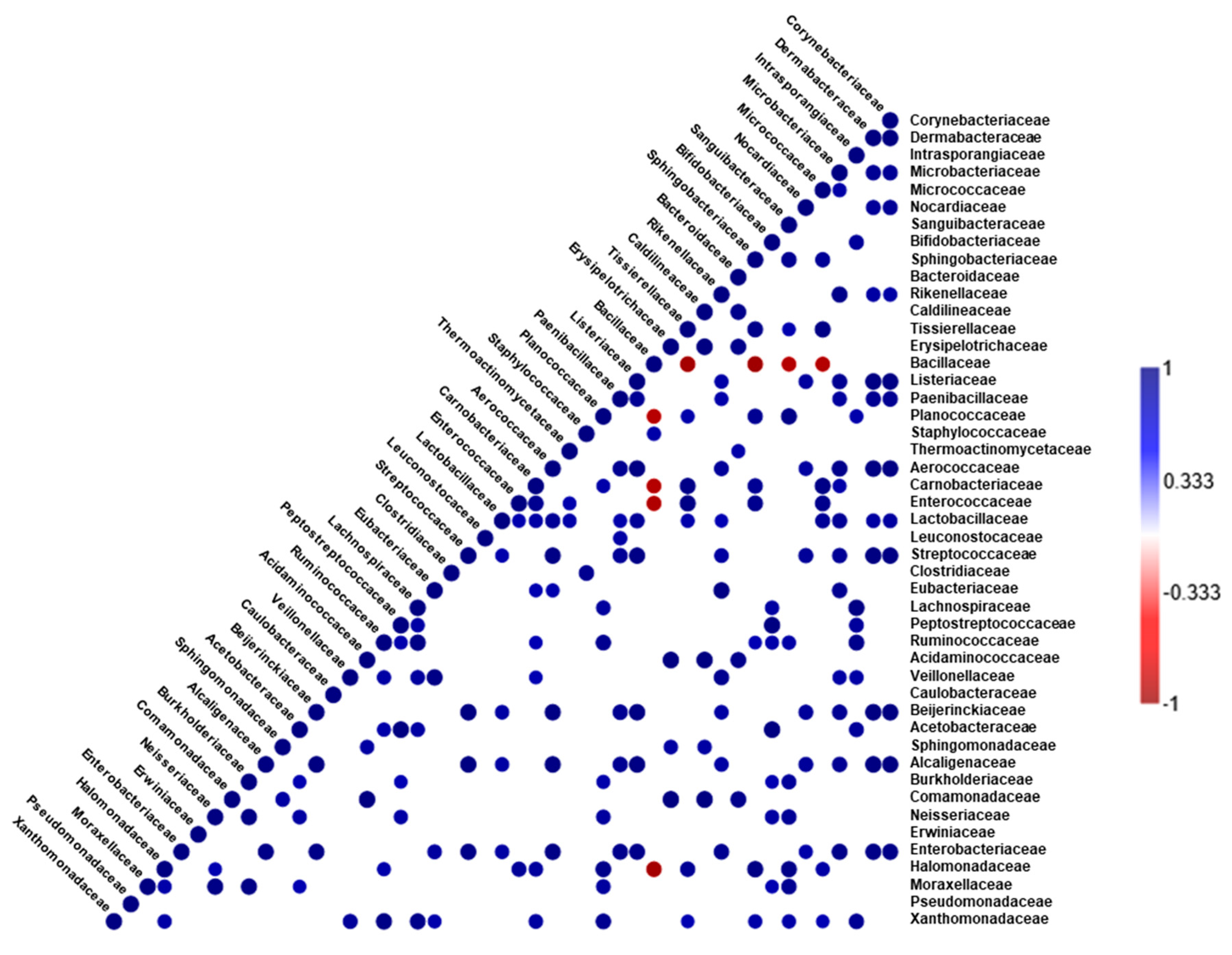
3.2. Microbial Structure and Dominant Taxa in Coriander and Control Soy Sauce
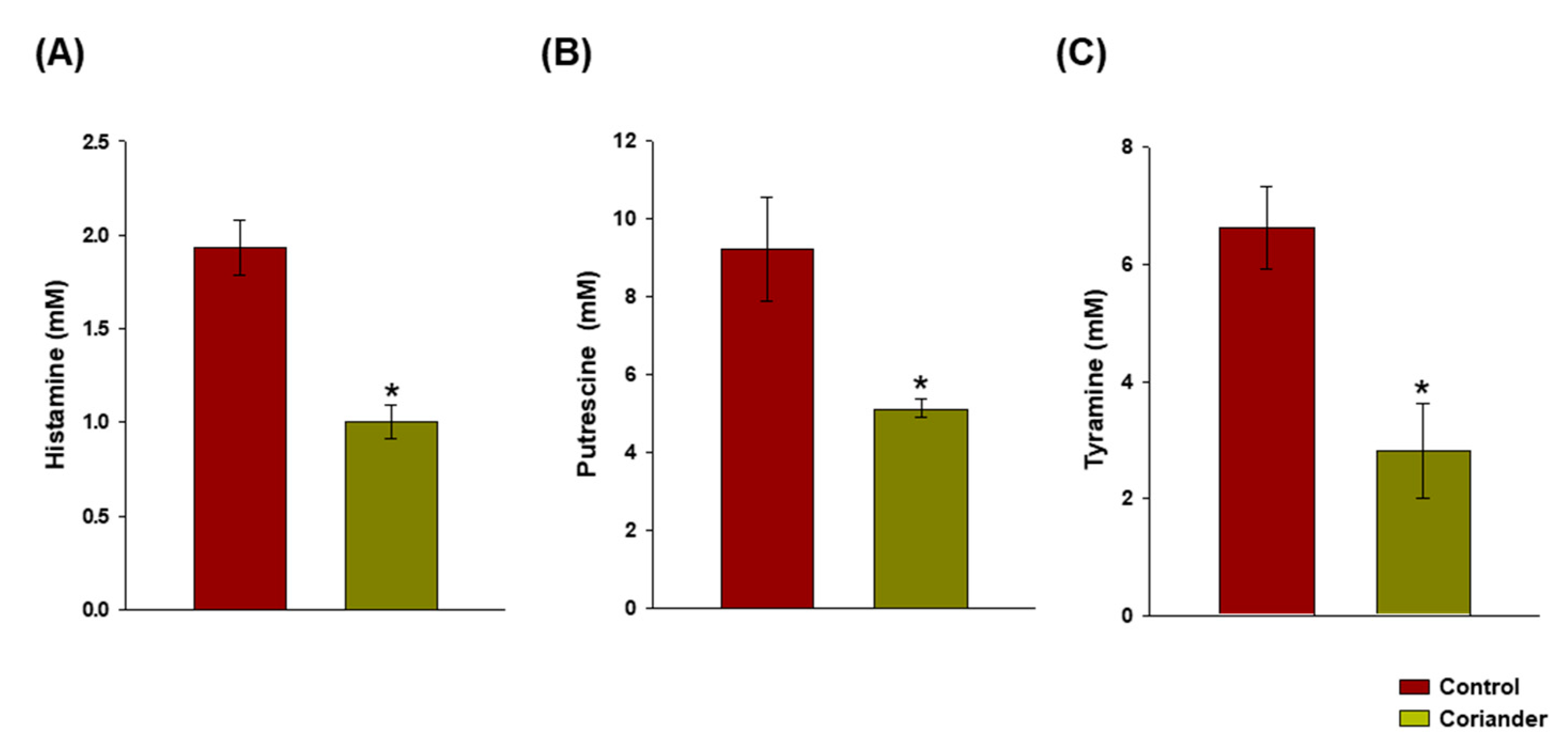
3.3. Reduction in the Biogenic Amine Content in Korean Soy Sauce upon Addition of Coriander during Fermentation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luh, B.S. Industrial production of soy sauce. J. Ind. Microbiol. 1995, 14, 467–471. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Xu, N.; Hu, Y.; Wang, C.; He, J.; Cao, Y.; Chen, S.; Li, D. Soy sauce classification by geographic region and fermentation based on artificial neural network and genetic algorithm. J. Agric. Food Chem. 2014, 62, 12294–12298. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Sohn, K.H. The changes of component in traditional Korean soy sauce during ripening period (I). Korean J. Soc. Food Sci. 1994, 10, 29–34. [Google Scholar]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Watanabe, I. Antiplatelet activity of soy sauce as functional seasoning. J. Agric. Food Chem. 1999, 47, 4167–4174. [Google Scholar] [CrossRef]
- Long, L.H.; Kwee, D.; Halliwell, B. The antioxidant activities of seasonings used in Asian cookings. Powerful antioxidant activity of dark soy sauce revealed using ABTS assay. Free Radic. Res. 2000, 32, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Hara-Kudo, Y.; Kumagai, S. Reduction of Escherichia coli O157:H7 populations by soy sauce: A fermented seasoning. J. Food Prot. 1998, 61, 657–661. [Google Scholar] [CrossRef]
- Ito, A.; Watanabe, H.; Basaran, N. Effects of soy products in reducing risk of spontaneous and neutron-induced liver tumors in mice. Int. J. Oncol. 1993, 2, 773–776. [Google Scholar] [CrossRef]
- Cho, K.M.; Seo, W.T. Bacterial diversity in a Korean traditional soybean fermented foods (doenjang and ganjang) by 16S rRNA gene sequence analysis. Food Sci. Biotechnol. 2007, 16, 320–324. [Google Scholar]
- Lee, K.E.; Lee, S.M.; Choi, Y.H.; Hurh, B.S.; Kim, Y.S. Comparative volatile profiles in soy sauce according to inoculated microorganisms. Biosci. Biotechnol. Biochem. 2013, 77, 2192–2200. [Google Scholar] [CrossRef] [Green Version]
- Yongmei, L.; Xiaohong, C.; Mei, J.; Xin, L.; Rahman, N.; Mingsheng, D.; Yan, G. Biogenic amines in Chinese soy sauce. Food Control 2009, 20, 593–597. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Lee, M.; Song, J.H.; Jung, M.Y.; Lee, S.H.; Chang, J.Y. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 2017, 66, 173–183. [Google Scholar] [CrossRef]
- Mannaa, M.; Seo, Y.S.; Park, I. Effect of seafood (gizzard shad) supplementation on the chemical composition and microbial dynamics of radish kimchi during fermentation. Sci. Rep. 2019, 9, 17693. [Google Scholar] [CrossRef]
- Han, D.M.; Chun, B.H.; Feng, T.; Kim, H.M.; Jeon, C.O. Dynamics of microbial communities and metabolites in ganjang, a traditional Korean fermented soy sauce, during fermentation. Food Microbiol. 2020, 92, 103591. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Huang, J.; Wu, X.; Fan, J.; Xu, Y.; Wu, C.; Jin, Y.; Zhou, R. Effect of raw material and starters on the metabolite constituents and microbial community diversity of fermented soy sauce. J. Sci. Food Agric. 2019, 99, 5687–5695. [Google Scholar] [CrossRef]
- Pereira, M.P.; Tavano, O.L. Use of different spices as potential natural antioxidant additives on cooked beans (Phaseolus vulgaris). Increase of DPPH radical scavenging activity and total phenolic content. Plant Foods Hum. Nutr. 2014, 69, 337–343. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef]
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M.; Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: Volatile and non volatile extracts. Int. J. Food Prop. 2012, 15, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Li, W.; Chang, Y. CD-HIT-OTU-MiSeq, an improved approach for clustering and analyzing paired end MiSeq 16S rRNA sequences. BioRxiv 2017, 153783. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Chun, B.H.; Jeon, C.O. Chromohalobacter is a causing agent for the production of organic acids and putrescine during fermentation of ganjang, a Korean traditional soy sauce. J. Food Sci. 2015, 80, 2853–2859. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.; Ryan, P.D. Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Xu, Y. Effects of Tetragenococcus halophilus and Candida versatilis on the production of aroma-active and umami-taste compounds during soy sauce fermentation. J. Sci. Food Agric. 2020, 100, 2782–2790. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [Green Version]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Potential of coriander (Coriandrum sativum) oil as a natural antimicrobial compound in controlling Campylobacter jejuni in raw meat. Biosci. Biotechnol. Biochem. 2010, 74, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Peçonek, J.; Gruber, C.; Gallego, V.; Ventosa, A.; Busse, H.J.; Kämpfer, P.; Radax, C.; Stan-Lotter, H. Reclassification of Pseudomonas beijerinckii Hof 1935 as Chromohalobacter beijerinckii comb. nov., and emended description of the species. Int. J. Sys. Evol. Microbiol. 2006, 56, 1953–1957. [Google Scholar] [CrossRef] [Green Version]
- Beutling, D.M.; Peçonek, J.; Stan-Lotter, H. Chromohalobacter beijerinckii: A psychrophilic, extremely halotolerant and enzymatically active microbe from salted food with the capacity for biogenic amine production. Eur. Food Res. Technol. 2009, 229, 725–730. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, K.; Sugiura, M. Soy sauce allergy. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 852–855. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [Green Version]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannaa, M.; Seo, Y.-S.; Park, I. Addition of Coriander during Fermentation of Korean Soy Sauce (Gangjang) Causes Significant Shift in Microbial Composition and Reduction in Biogenic Amine Levels. Foods 2020, 9, 1346. https://doi.org/10.3390/foods9101346
Mannaa M, Seo Y-S, Park I. Addition of Coriander during Fermentation of Korean Soy Sauce (Gangjang) Causes Significant Shift in Microbial Composition and Reduction in Biogenic Amine Levels. Foods. 2020; 9(10):1346. https://doi.org/10.3390/foods9101346
Chicago/Turabian StyleMannaa, Mohamed, Young-Su Seo, and Inmyoung Park. 2020. "Addition of Coriander during Fermentation of Korean Soy Sauce (Gangjang) Causes Significant Shift in Microbial Composition and Reduction in Biogenic Amine Levels" Foods 9, no. 10: 1346. https://doi.org/10.3390/foods9101346
APA StyleMannaa, M., Seo, Y.-S., & Park, I. (2020). Addition of Coriander during Fermentation of Korean Soy Sauce (Gangjang) Causes Significant Shift in Microbial Composition and Reduction in Biogenic Amine Levels. Foods, 9(10), 1346. https://doi.org/10.3390/foods9101346







