Proteomic Insights into the Biology of the Most Important Foodborne Parasites in Europe
Abstract
:1. Introduction
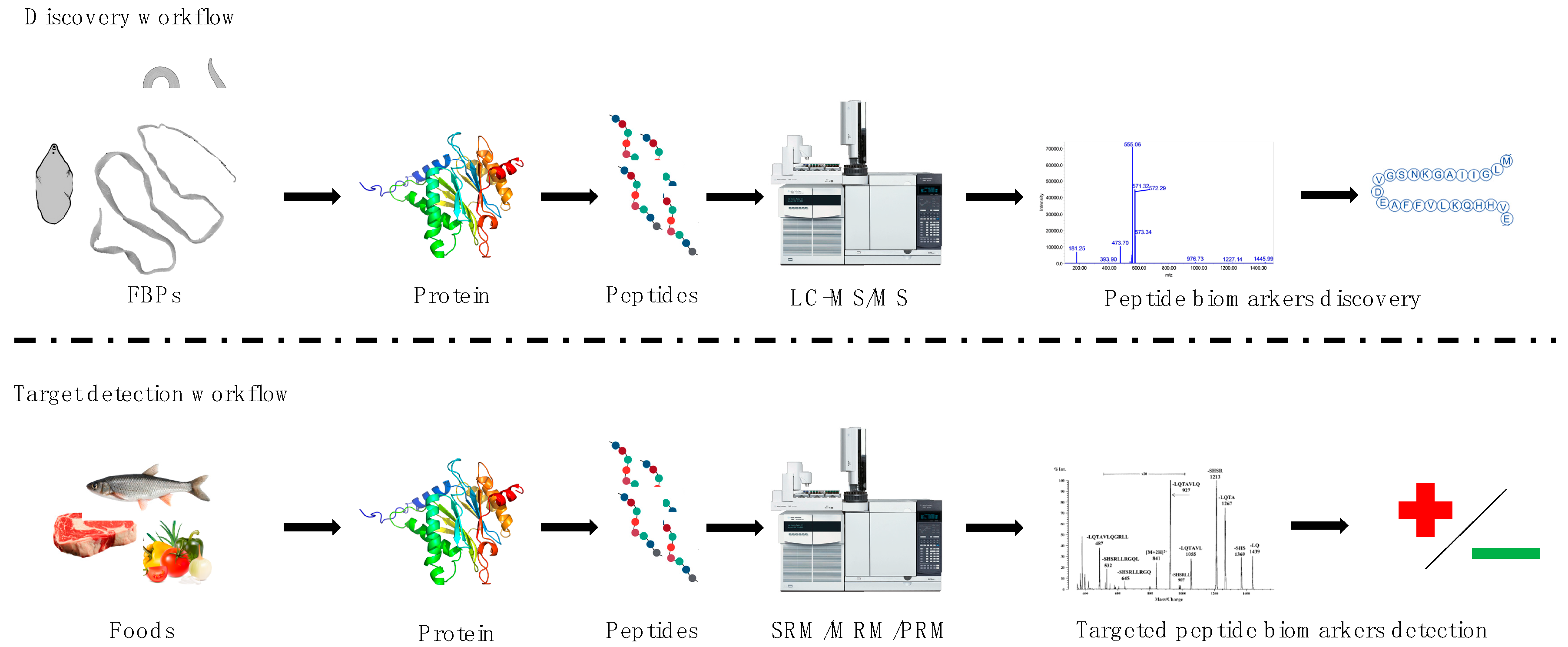
2. Discovery Approach—Description of the Selected FBPs and the Proteomics Methods Used to Study Them
2.1. Waterborne Parasitic Species
2.1.1. Cryptosporidium spp.
2.1.2. Giardia lamblia
2.1.3. Cyclospora cayetanensis
2.1.4. Entamoeba histolytica
2.1.5. Spirometra spp.
2.2. Soil- and Plant-Borne Parasitic Species
2.2.1. Echinococcus multiocularis and E. granulosus
2.2.2. Toxocara spp.
2.2.3. Ascaris spp.
2.2.4. Fasciola spp.
2.2.5. Trypanosoma cruzi
2.2.6. Trichuris trichiura
2.3. Meat-Borne Parasitic Species
2.3.1. Toxoplasma gondii
2.3.2. Trichinella spp.
2.3.3. Taenia spp.
2.3.4. Sarcocystis spp.
2.4. Seafood-Borne Parasitic Species
2.4.1. Opisthorchiidae
2.4.2. Angiostrongylus cantonesis
2.4.3. Diphyllobothrium spp.
2.4.4. Paragonimus spp.
2.4.5. Heterophyidae
3. Targeted Approach—Proteomics Methods Proposed to Use for Detection of Selected FBPs in Food
Anisakidae
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Trevisan, C.; Torgerson, P.R.; Robertson, L.J. Foodborne Parasites in Europe: Present Status and Future Trends. Trends Parasitol. 2019, 35, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozio, E. How globalization and climate change could affect foodborne parasites. Exp. Parasitol. 2020, 208, 107807. [Google Scholar] [CrossRef] [PubMed]
- Dorny, P.; Praet, N.; Deckers, N.; Gabriel, S. Emerging food-borne parasites. Vet. Parasitol. 2009, 163, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D. Zoonotic foodborne parasites and their surveillance. OIE Rev. Sci. Tech. 2013, 32, 559–569. [Google Scholar] [CrossRef] [Green Version]
- EFSA and ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16. [CrossRef]
- Poulin, R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Public health risks associated with food-borne parasites. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Crompton, D.W.T. How Much Human Helminthiasis Is There in the World? J. Parasitol. 1999, 85, 397–403. [Google Scholar] [CrossRef]
- European Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down specific rules on official controls for Trichinella in meat. Off. J. Eur. Union 2015, L212, 7–34.
- European Commission Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, 2003, 83.
- European Commission Regulation (EC) No 853/2004 of the European Parlamient and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L 139, 55.
- Åberg, R.; Sjöman, M.; Hemminki, K.; Pirnes, A.; Räsänen, S.; Kalanti, A.; Pohjanvirta, T.; Caccio, S.M.; Pihlajasaari, A.; Toikkanen, S.; et al. Cryptosporidium parvum Caused a Large Outbreak Linked to Frisée Salad in Finland, 2012. Zoonoses Public Health 2015, 62, 618–624. [Google Scholar] [CrossRef] [PubMed]
- McKerr, C.; Adak, G.K.; Nichols, G.; Gorton, R.; Chalmers, R.M.; Kafatos, G.; Cosford, P.; Charlett, A.; Reacher, M.; Pollock, K.G.; et al. An outbreak of cryptosporidium parvum across England and Scotland associated with consumption of fresh pre-cut salad leaves, May 2012. PLoS ONE 2015, 10, e0125955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKerr, C.; O’Brien, S.J.; Chalmers, R.M.; Vivancos, R.; Christley, R.M. Exposures associated with infection with Cryptosporidium in industrialised countries: A systematic review protocol. Syst. Rev. 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Robertson, L.J.; Dorny, P.; Jordan, S.; Kärssin, A.; Katzer, F.; La Carbona, S.; Lalle, M.; Lassen, B.; Mladineo, I.; et al. Parasite detection in food: Current status and future needs for validation. Trends Food Sci. Technol. 2020, 99, 337–350. [Google Scholar] [CrossRef]
- Cifuentes, A. Food Analysis and Foodomics. J Chromatogr. A. 2009, 1216, 7109. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, J.M.; Ortea, I.; Carrera, M. Proteomics and its applications for food authentication and food-technology research. Trends Anal. Chem. 2013, 52, 135–141. [Google Scholar] [CrossRef]
- Herrero, M.; Simó, C.; García-Cañas, V.; Ibáñez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2012, 31, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, R.A.; Uzzau, S.; Mazzeo, M.F. Editorial: Proteomics for studying foodborne microorganisms and their impact on food quality and human health. Front. Nutr. 2019, 6, 104. [Google Scholar] [CrossRef]
- Pandey, A.; Mann, M. Proteomics to study genes and genomes. Nature 2000, 405, 837–846. [Google Scholar] [CrossRef]
- Ong, S.E.; Mann, M. Mass Spectrometry–Based Proteomics Turns Quantitative. Nat. Chem. Biol. 2005, 1, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Emery-Corbin, S.J.; Grüttner, J.; Svärd, S. Transcriptomic and proteomic analyses of Giardia intestinalis: Intestinal epithelial cell interactions. In Advances in Parasitology; Academic Press: London, UK, 2020; Volume 107, pp. 139–171. ISBN 9780128204757. [Google Scholar]
- Walhout, M.; Vidal, M.; Dekker, J. Handbook of Systems Biology; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123859440. [Google Scholar]
- 50 Helminth Genomes Initiative. Available online: http://www.sanger.ac.uk/resources/downloads/helminths/ (accessed on 18 July 2020).
- Ginger, M.L.; McKean, P.G.; Burchmore, R.; Grant, K.M. Proteomic insights into parasite biology. Parasitology 2012, 139, 1101–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcilla, A.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Sánchez del Pino, M.M.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, F.; Cheng, G. Exosome-like vesicles of helminths: Implication of pathogenesis and vaccine development. Ann. Transl. Med. 2017, 5, 10–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Młocicki, D.; Sulima, A.; Bień, J.; Näreaho, A.; Zawistowska-Deniziak, A.; Basałaj, K.; Sałamatin, R.; Conn, D.B.; Savijoki, K. Immunoproteomics and Surfaceomics of the Adult Tapeworm Hymenolepis diminuta. Front. Immunol. 2018, 9, 2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, R.; Bernal, M.D.; Marcilla, A. Proteomics of foodborne trematodes. J. Proteom. 2011, 74, 1485–1503. [Google Scholar] [CrossRef]
- Stryiński, R.; Mateos, J.; Pascual, S.; González, Á.F.; Gallardo, J.M.; Łopieńska-Biernat, E.; Medina, I.; Carrera, M. Proteome profiling of L3 and L4 Anisakis simplex development stages by TMT-based quantitative proteomics. J. Proteom. 2019, 201, 1–11. [Google Scholar] [CrossRef]
- Prester, L. Seafood Allergy, Toxicity, and Intolerance: A Review. J. Am. Coll. Nutr. 2016, 35, 271–283. [Google Scholar] [CrossRef]
- Marzano, V.; Tilocca, B.; Fiocchi, A.G.; Vernocchi, P.; Levi Mortera, S.; Urbani, A.; Roncada, P.; Putignani, L. Perusal of food allergens analysis by mass spectrometry-based proteomics. J. Proteom. 2020, 215, 103636. [Google Scholar] [CrossRef]
- Carrera, M.; Piñeiro, C.; Martinez, I. Proteomic Strategies to Evaluate the Impact of Farming Conditions on Food Quality and Safety in Aquaculture Products. Foods 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Bensimon, A.; Collins, B.C.; Ludwig, C.; Sabido, E. Applications and Developments in Targeted Proteomics: From SRM to DIA/SWATH. Proteomics 2016, 16, 2065–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, U.; Hijjawi, N.; Xiao, L. Foodborne cryptosporidiosis. Int. J. Parasitol. 2018, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, O.; González-Díaz, M.; Garibay-Escobar, A.; Burgara-Estrella, A.; Cano, M.; Durazo, M.; Bernal, R.M.; Hernandez, J.; Xiao, L. Molecular Characterization of Cryptosporidium spp. in Children from Mexico. PLoS ONE 2014, 9, e96128. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, R.M.; Davies, A.P. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals—A one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Bones, A.J.; Jossé, L.; More, C.; Miller, C.N.; Michaelis, M.; Tsaousis, A.D. Past and future trends of Cryptosporidium in vitro research. Exp. Parasitol. 2019, 196, 28–37. [Google Scholar] [CrossRef]
- Sanderson, S.J.; Xia, D.; Prieto, H.; Yates, J.; Heiges, M.; Kissinger, J.C.; Bromley, E.; Lal, K.; Sinden, R.E.; Tomley, F.; et al. Determining the protein repertoire of Cryptosporidium parvum sporozoites. Proteomics 2008, 8, 1398–1414. [Google Scholar] [CrossRef] [Green Version]
- Siddiki, A.M.A.M.Z.; Wastling, J.M. Charting the proteome of Cryptosporidium parvum sporozoites using sequence similarity-based BLAST searching. J. Vet. Sci. 2009, 10, 203–210. [Google Scholar] [CrossRef]
- Snelling, W.J.; Lin, Q.; Moore, J.E.; Millar, B.C.; Tosini, F.; Pozio, E.; Dooley, J.S.G.; Lowery, C.J. Proteomics analysis and protein expression during sporozoite excystation of Cryptosporidium parvum (coccidia, apicomplexa). Mol. Cell. Proteom. 2007, 6, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.K.; Kumar, S.; Sahu, P.S.; Mahapatra, R.K. In silico identification and validation of a novel hypothetical protein in Cryptosporidium hominis and virtual screening of inhibitors as therapeutics. Parasitol. Res. 2017, 116, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ringqvist, E.; Palm, J.E.D.; Skarin, H.; Hehl, A.B.; Weiland, M.; Davids, B.J.; Reiner, D.S.; Griffiths, W.J.; Eckmann, L.; Gillin, F.D.; et al. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol. Biochem. Parasitol. 2008, 159, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubourg, A.; Xia, D.; Winpenny, J.P.; Al Naimi, S.; Bouzid, M.; Sexton, D.W.; Wastling, J.M.; Hunter, P.R.; Tyler, K.M. Giardia secretome highlights secreted tenascins as a key component of pathogenesis. Gigascience 2018, 7, giy003. [Google Scholar] [CrossRef]
- Ma’ayeh, S.Y.; Liu, J.; Peirasmaki, D.; Hörnaeus, K.; Bergström Lind, S.; Grabherr, M.; Bergquist, J.; Svärd, S.G. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells. PLoS Negl. Trop. Dis. 2017, 11, e0006120. [Google Scholar] [CrossRef] [Green Version]
- Wampfler, P.B.; Tosevski, V.; Nanni, P.; Spycher, C.; Hehl, A.B. Proteomics of Secretory and Endocytic Organelles in Giardia lamblia. PLoS ONE 2014, 9, e94089. [Google Scholar] [CrossRef] [Green Version]
- Evans-Osses, I.; Mojoli, A.; Monguió-Tortajada, M.; Marcilla, A.; Aran, V.; Amorim, M.; Inal, J.; Borràs, F.E.; Ramirez, M.I. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur. J. Cell Biol. 2017, 96, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Davids, B.J.; Liu, C.M.; Hanson, E.M.; Le, C.H.Y.; Ang, J.; Hanevik, K.; Fischer, M.; Radunovic, M.; Langeland, N.; Ferella, M.; et al. Identification of conserved candidate vaccine antigens in the surface proteome of giardia lamblia. Infect. Immun. 2019, 87, e00219-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Braga, S.; Uldry, A.C.; Heller, M.; Müller, N. Comparative proteomics of three Giardia lamblia strains: Investigation of antigenic variation in the post-genomic era. Parasitology 2020. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Cyclosporiasis. Available online: https://www.cdc.gov/parasites/cyclosporiasis/index.html (accessed on 22 July 2020).
- Tefera, T.; Tysnes, K.R.; Utaaker, K.S.; Robertson, L.J. Parasite contamination of berries: Risk, occurrence, and approaches for mitigation. Food Waterborne Parasitol. 2018, 10, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herwaldt, B.L. Cyclospora cayetanensis: A Review, Focusing on the Outbreaks of Cyclosporiasis in the 1990s. Clin. Infect. Dis. 2000, 31, 1040–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.J. Emerging and re-emerging parasitic diseases. J. Int. Med. Sci. Acad. 2010, 23, 45–50. [Google Scholar]
- Cinar, H.N.; Qvarnstrom, Y.; Wei-Pridgeon, Y.; Li, W.; Nascimento, F.S.; Arrowood, M.J.; Murphy, H.R.; Jang, A.; Kim, E.; Kim, R.; et al. Comparative sequence analysis of Cyclospora cayetanensis apicoplast genomes originating from diverse geographical regions. Parasit. Vectors 2016, 9, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, L.; Zheng, H.; Xu, Z.; Roellig, D.M.; Li, N.; Frace, M.A.; Tang, K.; Arrowood, M.J.; Moss, D.M.; et al. Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genom. 2016, 17, 316. [Google Scholar] [CrossRef] [Green Version]
- Luna-Nácar, M.; Navarrete-Perea, J.; Moguel, B.; Bobes, R.J.; Laclette, J.P.; Carrero, J.C. Proteomic Study of Entamoeba histolytica Trophozoites, Cysts, and Cyst-Like Structures. PLoS ONE 2016, 11, e0156018. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, R.; Lim, Y.A.L.; Amir, A. Medical Parasitology; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-68794-0. [Google Scholar]
- Leitsch, D.; Radauer, C.; Paschinger, K.; Wilson, I.B.H.; Breiteneder, H.; Scheiner, O.; Duchêne, M. Entamoeba histolytica: Analysis of the trophozoite proteome by two-dimensional polyacrylamide gel electrophoresis. Exp. Parasitol. 2005, 110, 191–195. [Google Scholar] [CrossRef]
- Tolstrup, J.; Krause, E.; Tannich, E.; Bruchhaus, I. Proteomic analysis of Entamoeba histolytica. Parasitology 2007, 134, 289–298. [Google Scholar] [CrossRef]
- Marion, S.; Laurent, C.; Guillén, N. Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: A proteomic approach. Cell. Microbiol. 2005, 7, 1504–1518. [Google Scholar] [CrossRef]
- Biller, L.; Matthiesen, J.; Kühne, V.; Lotter, H.; Handal, G.; Nozaki, T.; Saito-Nakano, Y.; Schümann, M.; Roeder, T.; Tannich, E.; et al. The Cell Surface Proteome of Entamoeba histolytica. Mol. Cell. Proteom. 2014, 13, 132–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Rosas, I.; Marchat, L.A.; Olvera, B.G.; Guillen, N.; Weber, C.; Hernández de la Cruz, O.; Ruíz-García, E.; Astudillo-de la Vega, H.; López-Camarillo, C. Proteomic analysis identifies endoribouclease EhL-PSP and EhRRP41 exosome protein as novel interactors of EhCAF1 deadenylase. J. Proteom. 2014, 111, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Ujang, J.; Sani, A.A.A.; Lim, B.H.; Noordin, R.; Othman, N. Analysis of Entamoeba histolytica Membrane Proteome Using Three Extraction Methods. Proteomics 2018, 18, 1700397. [Google Scholar] [CrossRef]
- Ujang, J.A.; Kwan, S.H.; Ismail, M.N.; Lim, B.H.; Noordin, R.; Othman, N. Proteome analysis of excretory-secretory proteins of Entamoeba histolytica HM1:IMSS via LC–ESI–MS/MS and LC–MALDI–TOF/TOF. Clin. Proteom. 2016, 13, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo, D.; Aït-Ammar, N.; Syan, S.; Sachse, M.; Jhingan, G.D.; Guillén, N. Cellular and proteomics analysis of the endomembrane system from the unicellular Entamoeba histolytica. J. Proteom. 2015, 112, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Leitsch, D.; Wilson, I.B.; Paschinger, K.; Duchêne, M. Comparison of the proteome profiles of Entamoeba histolytica and its close but non-pathogenic relative Entamoeba dispar. Wien. Klin. Wochenschr. 2006, 118, 37–41. [Google Scholar] [CrossRef]
- Davis, P.H.; Zhang, X.; Guo, J.; Townsend, R.R.; Stanley, S.L. Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Mol. Microbiol. 2006, 61, 1523–1532. [Google Scholar] [CrossRef]
- Davis, P.H.; Chen, M.; Zhang, X.; Clark, C.G.; Townsend, R.R.; Stanley, S.L. Proteomic Comparison of Entamoeba histolytica and Entamoeba dispar and the Role of E. histolytica Alcohol Dehydrogenase 3 in Virulence. PLoS Negl. Trop. Dis. 2009, 3, e415. [Google Scholar] [CrossRef]
- Calderaro, A.; Piergianni, M.; Buttrini, M.; Montecchini, S.; Piccolo, G.; Gorrini, C.; Rossi, S.; Chezzi, C.; Arcangeletti, M.C.; Medici, M.C.; et al. MALDI-TOF Mass Spectrometry for the Detection and Differentiation of Entamoeba histolytica and Entamoeba dispar. PLoS ONE 2015, 10, e0122448. [Google Scholar] [CrossRef]
- Ali, I.K.M.; Haque, R.; Siddique, A.; Kabir, M.; Sherman, N.E.; Gray, S.A.; Cangelosi, G.A.; Petri, W.A. Proteomic Analysis of the Cyst Stage of Entamoeba histolytica. PLoS Negl. Trop. Dis. 2012, 6, e1643. [Google Scholar] [CrossRef]
- Marquay Markiewicz, J.; Syan, S.; Hon, C.C.; Weber, C.; Faust, D.; Guillen, N. A Proteomic and Cellular Analysis of Uropods in the Pathogen Entamoeba histolytica. PLoS Negl. Trop. Dis. 2011, 5, e1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention Sparganosis. Available online: https://www.cdc.gov/dpdx/sparganosis/index.html (accessed on 28 July 2020).
- Wiwanitkit, V. A review of human sparganosis in Thailand. Int. J. Infect. Dis. 2005, 9, 312–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, Y.J.; Sohn, W.M.; Bae, Y.M.; Hong, S.T.; Choi, M.H. Differential protein expression in Spirometra erinacei according to its development in its final host. Parasitol. Res. 2009, 105, 1549–1556. [Google Scholar] [CrossRef]
- Hu, D.D.; Cui, J.; Wang, L.; Liu, L.N.; Wei, T.; Wang, Z.Q. Immunoproteomic analysis of the excretory-secretory proteins from Spirometra mansoni sparganum. Iran. J. Parasitol. 2013, 8, 408–416. [Google Scholar]
- Hu, D.D.; Cui, J.; Xiao, D.; Wang, L.; Liu, L.N.; Liu, R.D.; Zhang, J.Z.; Wang, Z.Q. Identification of early diagnostic antigens from Spirometra erinaceieuropaei sparganum soluble proteins using immunoproteomics. Southeast Asian J. Trop. Med. Public Health 2014, 45, 576–583. [Google Scholar] [PubMed]
- Liu, W.; Tang, H.; Abuzeid, A.M.I.; Tan, L.; Wang, A.; Wan, X.; Zhang, H.; Liu, Y.; Li, G. Protein phosphorylation networks in spargana of Spirometra erinaceieuropaei revealed by phosphoproteomic analysis. Parasites Vectors 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, P.; Schantz, P.M. Echinococcosis: A review. Int. J. Infect. Dis. 2009, 13, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moro, P.L.; Garcia, H.H.; Gonzales, A.E.; Bonilla, J.J.; Verastegui, M.; GilmanMD, R.H. Screening for cystic echinococcosis in an endemic region of Peru using portable ultrasonography and the enzyme-linked immunoelectrotransfer blot (EITB) assay. Parasitol. Res. 2005, 96, 242–246. [Google Scholar] [CrossRef]
- WHO Informal Working Group on Echinococcosis (IWGE) Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull. World Health Organ. 1996, 74, 231–242.
- WHO Informal Working Group on Echinococcosis (IWGE). Puncture, Aspiration, Injection, Re-aspiration: An Option for the Treatment of Cystic Echinococcosis; World Health Organization: Geneva, Switzerland, 2001; WHO/CDS/CSR/APH/2001.6. [Google Scholar]
- Chemale, G.; Van Rossum, A.J.; Jefferies, J.R.; Barrett, J.; Brophy, P.M.; Ferreira, H.B.; Zaha, A. Proteomic analysis of the larval stage of the parasite Echinococcus granulosus: Causative agent of cystic hydatid disease. Proteomics 2003, 3, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Kouguchi, H.; Matsumoto, J.; Katoh, Y.; Suzuki, T.; Oku, Y.; Yagi, K. Echinococcus multilocularis: Two-dimensional Western blotting method for the identification and expression analysis of immunogenic proteins in infected dogs. Exp. Parasitol. 2010, 124, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Z.; Lu, X.; Tang, C. Echinococcus multilocularis: Proteomic analysis of the protoscoleces by two-dimensional electrophoresis and mass spectrometry. Exp. Parasitol. 2009, 123, 162–167. [Google Scholar] [CrossRef]
- Hidalgo, C.; García, M.P.; Stoore, C.; Ramírez, J.P.; Monteiro, K.M.; Hellman, U.; Zaha, A.; Ferreira, H.B.; Galanti, N.; Landerer, E.; et al. Proteomics analysis of Echinococcus granulosus protoscolex stage. Vet. Parasitol. 2016, 218, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Manterola, C.; García, N.; Rojas, C. Aspectos Generales del Perfil Proteómico del Echinococcus granulosus. Int. J. Morphol. 2019, 37, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Miles, S.; Portela, M.; Cyrklaff, M.; Ancarola, M.E.; Frischknecht, F.; Durán, R.; Dematteis, S.; Mourglia-Ettlin, G. Combining proteomics and bioinformatics to explore novel tegumental antigens as vaccine candidates against Echinococcus granulosus infection. J. Cell. Biochem. 2019, 120, 15320–15336. [Google Scholar] [CrossRef]
- Monteiro, K.M.; De Carvalho, M.O.; Zaha, A.; Ferreira, H.B. Proteomic analysis of the Echinococcus granulosus metacestode during infection of its intermediate host. Proteomics 2010, 10, 1985–1999. [Google Scholar] [CrossRef]
- Longuespée, R.; Casadonte, R.; Kriegsmann, M.; Wandernoth, P.; Lisenko, K.; Mazzucchelli, G.; Becker, M.; Kriegsmann, J. Proteomic investigation of human cystic echinococcosis in the liver. Mol. Biochem. Parasitol. 2017, 211, 9–14. [Google Scholar] [CrossRef]
- Ahn, C.S.; Kim, J.G.; Han, X.; Kang, I.; Kong, Y. Comparison of Echinococcus multilocularis and Echinococcus granulosus hydatid fluid proteome provides molecular strategies for specialized host-parasite interactions. Oncotarget 2017, 8, 97009–97024. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.J.; Xu, L.L.; Zhang, T.; Xu, M.; Yao, J.; Fang, C.Y.; Feng, Z.; Yang, P.Y.; Hu, W.; Liu, F. Proteomic characterization of larval and adult developmental stages in Echinococcus granulosus reveals novel insight into host-parasite interactions. J. Proteom. 2013, 84, 158–175. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Cui, F.; Shi, C.; Ma, Y.; Yu, Y.; Zhao, W.; Zhao, J. Extracellular vesicles derived from Echinococcus granulosus hydatid cyst fluid from patients: Isolation, characterization and evaluation of immunomodulatory functions on T cells. Int. J. Parasitol. 2019, 49, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Ma, G.; Wang, T.; Koehler, A.V.; Hofmann, A.; Chang, B.C.H.; Macpherson, C.N.; Gasser, R.B. Human toxocariasis—A look at a neglected disease through an epidemiological ‘prism’. Infect. Genet. Evol. 2019, 74, 104002. [Google Scholar] [CrossRef] [PubMed]
- Schnieder, T.; Laabs, E.M.; Welz, C. Larval development of Toxocara canis in dogs. Vet. Parasitol. 2011, 175, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Korhonen, P.K.; Cai, H.; Young, N.D.; Nejsum, P.; Von Samson-Himmelstjerna, G.; Boag, P.R.; Tan, P.; Li, Q.; Min, J.; et al. Genetic blueprint of the zoonotic pathogen Toxocara canis. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, M.B.; Oviedo, Y.; Cooper, P.J.; Pacheco, L.G.C.; Pinheiro, C.S.; Ferreira, F.; Briza, P.; Alcantara-Neves, N.M. The somatic proteins of Toxocara canis larvae and excretory-secretory products revealed by proteomics. Vet. Parasitol. 2018, 259, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Sperotto, R.L.; Kremer, F.S.; Aires Berne, M.E.; Costa de Avila, L.F.; da Silva Pinto, L.; Monteiro, K.M.; Caumo, K.S.; Ferreira, H.B.; Berne, N.; Borsuk, S. Proteomic analysis of Toxocara canis excretory and secretory (TES) proteins. Mol. Biochem. Parasitol. 2017, 211, 39–47. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Dold, C.; Holland, C.V. Ascaris and ascariasis. Microbes Infect. 2011, 13, 632–637. [Google Scholar] [CrossRef]
- De Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef]
- Mrozińska-Gogol, J. Ascaris lumbricoides. In Medical Parasitology [Parazytologia medyczna]; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2016; pp. 174–180. ISBN 978-83-200-5138-4. [Google Scholar]
- Xu, M.J.; Fu, J.H.; Zhou, D.H.; Elsheikha, H.M.; Hu, M.; Lin, R.Q.; Peng, L.F.; Song, H.Q.; Zhu, X.Q. Ascaris lumbricoides and Ascaris suum: Comparative proteomic studies using 2-DE coupled with mass spectrometry. Int. J. Mass Spectrom. 2013, 339, 1–6. [Google Scholar] [CrossRef]
- Abebe, W.; Tsuji, N.; Kasuga-Aoki, H.; Miyoshi, T.; Isobe, T.; Arakawa, T.; Matsumoto, Y.; Yoshihara, S. Species-specific proteins identified in Ascaris lumbricoides and Ascaris suum using two-dimensional electrophoresis. Parasitol. Res. 2002, 88, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Nagorny, S.A.; Aleshukina, A.V.; Aleshukina, I.S.; Ermakova, L.A.; Pshenichnaya, N.Y. The application of proteomic methods (MALDI-toff MS) for studying protein profiles of some nematodes (dirofilaria and ascaris) for differentiating species. Int. J. Infect. Dis. 2019, 82, 61–65. [Google Scholar] [CrossRef] [Green Version]
- González-Miguel, J.; Morchón, R.; Gussoni, S.; Bossetti, E.; Hormaeche, M.; Kramer, L.H.; Simón, F. Immunoproteomic approach for identification of Ascaris suum proteins recognized by pigs with porcine ascariasis. Vet. Parasitol. 2014, 203, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chehayeb, J.F.; Robertson, A.P.; Martin, R.J.; Geary, T.G. Proteomic Analysis of Adult Ascaris suum Fluid Compartments and Secretory Products. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.P.; Fromm, B.; Andersen, S.D.; Marcilla, A.; Andersen, K.L.; Borup, A.; Williams, A.R.; Jex, A.R.; Gasser, R.B.; Young, N.D.; et al. Exploration of extracellular vesicles from Ascaris suum provides evidence of parasite–host cross talk. J. Extracell. Vesicles 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.W.; Dalton, J.P. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 2005, 35, 1255–1278. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Plant-Borne Trematode Zoonoses: Fascioliasis and Fasciolopsiasis. In Food-Borne Parasitic Zoonoses: Fish and Plant-Borne Parasites; Murrell, D.K., Fried, B., Eds.; Springer: New York, NY, USA, 2007; pp. 293–334. [Google Scholar]
- Mas-Coma, S. Human Fascoliasis: Epidemiological patterns in human endemic areas of South America, Africa and Asia. Southeast Asian J. Trop. Med. Public Health 2004, 35, 1–11. [Google Scholar]
- Keiser, J.; Utzinger, J. Emerging Foodborne Trematodiasis. Emerg. Infect. Dis. 2005, 11, 1507–1514. [Google Scholar] [CrossRef]
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Irving, D.O.; Howell, M.J. Characterization of excretory-secretory antigens of Fasciola hepatica. Parasitology 1982, 85, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.P.; Tom, T.D.; Strand, M. Fasciola hepatica: Comparison of immature and mature immunoreactive glycoproteins. Parasite Immunol. 1985, 7, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Zimmerman, G.L.; Bishop, J.K. Host influence on the banding profiles of whole-body protein and excretory-secretory product of Fasciola hepatica (trematoda) by isoelectric focusing. Vet. Parasitol. 1992, 41, 57–68. [Google Scholar] [CrossRef]
- Lee, C.G.; Zimmerman, G.L.; Mulrooney, D.M. Isoelectric focusing of soluble proteins from Fasciola hepatica L, 1758 and Fascioloides magna B, 1875. Am. J. Vet. Res. 1992, 53, 246–250. [Google Scholar] [PubMed]
- Jefferies, J.R.; Brophy, P.M.; Barrett, J. Investigation of Fasciola hepatica sample preparation for two-dimensional electrophoresis. Electrophoresis 2000, 21, 3724–3729. [Google Scholar] [CrossRef]
- Jefferies, J.R.; Campbell, A.M.; van Rossum, A.J.; Barrett, J.; Brophy, P.M. Proteomic analysis of Fasciola hepatica excretory-secretory products. Proteomics 2001, 1, 1128–1132. [Google Scholar] [CrossRef]
- Chemale, G.; Morphew, R.; Moxon, J.V.; Morassuti, A.L.; LaCourse, E.J.; Barrett, J.; Johnston, D.A.; Brophy, P.M. Proteomic analysis of glutathione transferases from the liver fluke parasite, Fasciola hepatica. Proteomics 2006, 6, 6263–6273. [Google Scholar] [CrossRef]
- Robinson, M.W.; Tort, J.F.; Lowther, J.; Donnelly, S.M.; Wong, E.; Xu, W.; Stack, C.M.; Padula, M.; Herbert, B.; Dalton, J.P. Proteomics and Phylogenetic Analysis of the Cathepsin L Protease Family of the Helminth Pathogen Fasciola hepatica. Mol. Cell. Proteom. 2008, 7, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Marcilla, A.; De la Rubia, J.E.; Sotillo, J.; Bernal, D.; Carmona, C.; Villavicencio, Z.; Acosta, D.; Tort, J.; Bornay, F.J.; Esteban, J.G.; et al. Leucine Aminopeptidase Is an Immunodominant Antigen of Fasciola hepatica Excretory and Secretory Products in Human Infections. Clin. Vaccine Immunol. 2008, 15, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Morphew, R.M.; Wright, H.A.; LaCourse, E.J.; Porter, J.; Barrett, J.; Woods, D.J.; Brophy, P.M. Towards Delineating Functions within the Fasciola Secreted Cathepsin L Protease Family by Integrating In Vivo Based Sub-Proteomics and Phylogenetics. PLoS Negl. Trop. Dis. 2011, 5, e937. [Google Scholar] [CrossRef] [Green Version]
- Morphew, R.M.; Eccleston, N.; Wilkinson, T.J.; McGarry, J.; Perally, S.; Prescott, M.; Ward, D.; Williams, D.; Paterson, S.; Raman, M.; et al. Proteomics and in Silico Approaches To Extend Understanding of the Glutathione Transferase Superfamily of the Tropical Liver Fluke Fasciola gigantica. J. Proteome Res. 2012, 11, 5876–5889. [Google Scholar] [CrossRef]
- Morphew, R.M.; Hamilton, C.M.; Wright, H.A.; Dowling, D.J.; O’Neill, S.M.; Brophy, P.M. Identification of the major proteins of an immune modulating fraction from adult Fasciola hepatica released by Nonidet P40. Vet. Parasitol. 2013, 191, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morphew, R.M.; Wilkinson, T.J.; Mackintosh, N.; Jahndel, V.; Paterson, S.; McVeigh, P.; Abbas Abidi, S.M.; Saifullah, K.; Raman, M.; Ravikumar, G.; et al. Exploring and Expanding the Fatty-Acid-Binding Protein Superfamily in Fasciola Species. J. Proteome Res. 2016, 15, 3308–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cwiklinski, K.; de la Torre-Escudero, E.; Trelis, M.; Bernal, D.; Dufresne, P.J.; Brennan, G.P.; O’Neill, S.; Tort, J.; Paterson, S.; Marcilla, A.; et al. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell. Proteom. 2015, 14, 3258–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Maggio, L.S.; Tirloni, L.; Pinto, A.F.M.; Diedrich, J.K.; Yates III, J.R.; Benavides, U.; Carmona, C.; da Silva Vaz, I.; Berasain, P. Across intra-mammalian stages of the liver f luke Fasciola hepatica: A proteomic study. Sci. Rep. 2016, 6, 32796. [Google Scholar] [CrossRef] [Green Version]
- Chemale, G.; Perally, S.; LaCourse, E.J.; Prescott, M.C.; Jones, L.M.; Ward, D.; Meaney, M.; Hoey, E.; Brennan, G.P.; Fairweather, I.; et al. Comparative Proteomic Analysis of Triclabendazole Response in the Liver Fluke Fasciola hepatica. J. Proteome Res. 2010, 9, 4940–4951. [Google Scholar] [CrossRef]
- Morphew, R.M.; MacKintosh, N.; Hart, E.H.; Prescott, M.; LaCourse, E.J.; Brophy, P.M. In vitro biomarker discovery in the parasitic flatworm Fasciola hepatica for monitoring chemotherapeutic treatment. EuPA Open Proteom. 2014, 3, 85–99. [Google Scholar] [CrossRef]
- Moxon, J.V.; LaCourse, E.J.; Wright, H.A.; Perally, S.; Prescott, M.C.; Gillard, J.L.; Barrett, J.; Hamilton, J.V.; Brophy, P.M. Proteomic analysis of embryonic Fasciola hepatica: Characterization and antigenic potential of a developmentally regulated heat shock protein. Vet. Parasitol. 2010, 169, 62–75. [Google Scholar] [CrossRef]
- Wilson, R.A.; Wright, J.M.; de Castro-Borges, W.; Parker-Manuel, S.J.; Dowle, A.A.; Ashton, P.D.; Young, N.D.; Gasser, R.B.; Spithill, T.W. Exploring the Fasciola hepatica tegument proteome. Int. J. Parasitol. 2011, 41, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Haçarız, O.; Sayers, G.; Baykal, A.T. A Proteomic Approach To Investigate the Distribution and Abundance of Surface and Internal Fasciola hepatica Proteins during the Chronic Stage of Natural Liver Fluke Infection in Cattle. J. Proteome Res. 2012, 11, 3592–3604. [Google Scholar] [CrossRef]
- Ley, V.; Andrews, N.W.; Robbins, E.S.; Nussenzweig, V. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J. Exp. Med. 1988, 168, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, F.A.; Weirauch, C.; Felix, M.; Lazoski, C.; Abad-Franch, F. Evolution, Systematics, and Biogeography of the Triatominae, Vectors of Chagas Disease. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 265–344. [Google Scholar]
- Berry, A.S.F.; Salazar-Sánchez, R.; Castillo-Neyra, R.; Borrini-Mayorí, K.; Chipana-Ramos, C.; Vargas-Maquera, M.; Ancca-Juarez, J.; Náquira-Velarde, C.; Levy, M.Z.; Brisson, D. Sexual reproduction in a natural Trypanosoma cruzi population. PLoS Negl. Trop. Dis. 2019, 13, e0007392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.A.; Weatherly, D.B.; Minning, T.A.; Bundy, B.; Cavola, C.; Opperdoes, F.R.; Orlando, R.; Tarleton, R.L. The Trypanosoma cruzi Proteome. Science 2005, 309, 473–476. [Google Scholar] [CrossRef]
- Brunoro, G.V.F.; Caminha, M.A.; Ferreira, A.T.; da, S..; da Veiga Leprevost, F.; Carvalho, P.C.; Perales, J.; Valent, R.H.; Menna-Barreto, R.F.S. dReevaluating the Trypanosoma cruzi proteomic map: The shotgun description of bloodstream trypomastigotes. J. Proteom. 2015, 115, 58–65. [Google Scholar] [CrossRef]
- Nakayasu, E.S.; Sobreira, T.J.P.; Torres, R.; Ganiko, L.; Oliveira, P.S.L.; Marques, A.F.; Almeida, I.C. Improved Proteomic Approach for the Discovery of Potential Vaccine Targets in Trypanosoma cruzi. J. Proteome Res. 2012, 11, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Parodi-Talice, A.; Monteiro-Goes, V.; Arrambide, N.; Avila, A.R.; Duran, R.; Correa, A.; Dallagiovanna, B.; Cayota, A.; Krieger, M.; Goldenberg, S.; et al. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J. Mass Spectrom. 2007, 42, 1422–1432. [Google Scholar] [CrossRef]
- Amorim, J.C.; Batista, M.; da Cunha, E.S.; Lucena, A.C.R.; de Paula Lima, C.V.; Sousa, K.; Krieger, M.A.; Marchini, F.K. Quantitative proteome and phosphoproteome analyses highlight the adherent population during Trypanosoma cruzi metacyclogenesis. Sci. Rep. 2017, 7, 9899. [Google Scholar] [CrossRef] [Green Version]
- Lucena, A.C.R.; Amorim, J.C.; de Paula Lima, C.V.; Batista, M.; Krieger, M.A.; de Godoy, L.M.F.; Marchini, F.K. Quantitative phosphoproteome and proteome analyses emphasize the influence of phosphorylation events during the nutritional stress of Trypanosoma cruzi: The initial moments of in vitro metacyclogenesis. Cell Stress Chaperones 2019, 24, 927–936. [Google Scholar] [CrossRef]
- Avila, C.; Mule, S.; Rosa-Fernandes, L.; Viner, R.; Barisón, M.; Costa-Martins, A.; Oliveira, G.; Teixeira, M.; Marinho, C.; Silber, A.; et al. Proteome-Wide Analysis of Trypanosoma cruzi Exponential and Stationary Growth Phases Reveals a Subcellular Compartment-Specific Regulation. Genes 2018, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.L.; Contreras, V.T.; Marliére, N.P.; Aparecida Guarneri, A.; Villamizar Silva, L.H.; Mazzarotto, G.A.C.A.; Batista, M.; Soccol, V.T.; Krieger, M.A.; Probst, C.M. Recently differentiated epimastigotes from Trypanosoma cruzi are infective to the mammalian host. Mol. Microbiol. 2017, 104, 712–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, R.M.L.; Charneau, S.; Mandacaru, S.C.; Schwämmle, V.; Lima, B.D.; Roepstorff, P.; Ricart, C.A.O. Quantitative Proteomic and Phosphoproteomic Analysis of Trypanosoma cruzi Amastigogenesis. Mol. Cell. Proteom. 2014, 13, 3457–3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, N.O.; de Souza, R.T.; Cordero, E.M.; Maldonado, D.C.; Cortez, C.; Marini, M.M.; Ferreira, E.R.; Bayer-Santos, E.; de Almeida, I.C.; Yoshida, N.; et al. Molecular Characterization of a Novel Family of Trypanosoma cruzi Surface Membrane Proteins (TcSMP) Involved in Mammalian Host Cell Invasion. PLoS Negl. Trop. Dis. 2015, 9, e0004216. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.S.; Bancroft, A.J.; Goldrick, M.; Portsmouth, C.; Roberts, I.S.; Grencis, R.K. Exploitation of the Intestinal Microflora by the Parasitic Nematode Trichuris muris. Science 2010, 328, 1391–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention Parasites—Trichuriasis (Also Known as Whipworm Infection). Available online: https://www.cdc.gov/parasites/whipworm/ (accessed on 22 July 2020).
- Lillywhite, J.E.; Cooper, E.S.; Needham, C.S.; Venugopal, S.; Bundy, D.A.P.; Bianco, A.E. Identification and characterization of excreted/secreted products of Trichuris trichiura. Parasite Immunol. 1995, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.; Marcilla, P.; Kelly, P.; Vandenplas, M.; Osuna, A.; Trelis, M. Proteomic Analysis of Trichuris Trichiura Egg Extract Reveals Potential Immunomodulators and Diagnostic Targets. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Hurst, R.J.M.; Else, K.J. Trichuris muris research revisited: A journey through time. Parasitology 2013, 140, 1325–1339. [Google Scholar] [CrossRef] [Green Version]
- Eichenberger, R.M.; Talukder, M.H.; Field, M.A.; Wangchuk, P.; Giacomin, P.; Loukas, A.; Sotillo, J. Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication. J. Extracell. Vesicles 2018, 7, 1428004. [Google Scholar] [CrossRef] [Green Version]
- Tritten, L.; Tam, M.; Vargas, M.; Jardim, A.; Stevenson, M.M.; Keiser, J.; Geary, T.G. Excretory/secretory products from the gastrointestinal nematode Trichuris muris. Exp. Parasitol. 2017, 178, 30–36. [Google Scholar] [CrossRef]
- Shears, R.K.; Bancroft, A.J.; Sharpe, C.; Grencis, R.K.; Thornton, D.J. Vaccination Against Whipworm: Identification of Potential Immunogenic Proteins in Trichuris muris Excretory/Secretory Material. Sci. Rep. 2018, 8, 4508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2016; Volume 3, ISBN 9780429092954. [Google Scholar]
- Kijlstra, A.; Jongert, E. Toxoplasma-safe meat: Close to reality? Trends Parasitol. 2009, 25, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.M.; Rumpel, K.; Coombs, G.H.; Wastling, J.M. Characterisation of global protein expression by two-dimensional electrophoresis and mass spectrometry: Proteomics of Toxoplasma gondii. Int. J. Parasitol. 2002, 32, 39–51. [Google Scholar] [CrossRef]
- Nischik, N.; Schade, B.; Dytnerska, K.; Długońska, H.; Reichmann, G.; Fischer, H.G. Attenuation of mouse-virulent Toxoplasma gondii parasites is associated with a decrease in interleukin-12-inducing tachyzoite activity and reduced expression of actin, catalase and excretory proteins. Microbes Infect. 2001, 3, 689–699. [Google Scholar] [CrossRef]
- Wastling, J.M.; Xia, D. Proteomics of Toxoplasma gondii. In Toxoplasma Gondii: The Model Apicomplexan—Perspectives and Methods: Second Edition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 731–754. ISBN 9780123964816. [Google Scholar]
- Xia, D.; Sanderson, S.J.; Jones, A.R.; Prieto, J.H.; Yates, J.R.; Bromley, E.; Tomley, F.M.; Lal, K.; Sinden, R.E.; Brunk, B.P.; et al. The proteome of Toxoplasma gondii: Integration with the genome provides novel insights into gene expression and annotation. Genome Biol. 2008, 9, R116. [Google Scholar] [CrossRef] [Green Version]
- Dybas, J.M.; Madrid-Aliste, C.J.; Che, F.Y.; Nieves, E.; Rykunov, D.; Angeletti, R.H.; Weiss, L.M.; Kim, K.; Fiser, A. Computational Analysis and Experimental Validation of Gene Predictions in Toxoplasma gondii. PLoS ONE 2008, 3, e3899. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.H.; Zhao, F.R.; Nisbet, A.J.; Xu, M.J.; Song, H.Q.; Lin, R.Q.; Huang, S.Y.; Zhu, X.Q. Comparative proteomic analysis of different Toxoplasma gondii genotypes by two-dimensional fluorescence difference gel electrophoresis combined with mass spectrometry. Electrophoresis 2014, 35, 533–545. [Google Scholar] [CrossRef]
- Ma, G.Y.; Zhang, J.Z.; Yin, G.R.; Zhang, J.H.; Meng, X.L.; Zhao, F. Toxoplasma gondii: Proteomic analysis of antigenicity of soluble tachyzoite antigen. Exp. Parasitol. 2009, 122, 41–46. [Google Scholar] [CrossRef]
- Krishna, R.; Xia, D.; Sanderson, S.; Shanmugasundram, A.; Vermont, S.; Bernal, A.; Daniel-Naguib, G.; Ghali, F.; Brunk, B.P.; Roos, D.S.; et al. A large-scale proteogenomics study of apicomplexan pathogens-Toxoplasma gondii and Neospora caninum. Proteomics 2015, 15, 2618–2628. [Google Scholar] [CrossRef] [Green Version]
- Fritz, H.M.; Bowyer, P.W.; Bogyo, M.; Conrad, P.A.; Boothroyd, J.C. Proteomic Analysis of Fractionated Toxoplasma Oocysts Reveals Clues to Their Environmental Resistance. PLoS ONE 2012, 7, e29955. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.X.; Zhu, X.Q.; Elsheikha, H.M.; He, S.; Li, Q.; Zhou, D.H.; Suo, X. Global iTRAQ-based proteomic profiling of Toxoplasma gondii oocysts during sporulation. J. Proteom. 2016, 148, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Kafsack, B.F.C.; Cole, R.N.; Beckett, P.; Shen, R.F.; Carruthers, V.B. The Opportunistic Pathogen Toxoplasma gondii Deploys a Diverse Legion of Invasion and Survival Proteins. J. Biol. Chem. 2005, 280, 34233–34244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.K.; Ahn, H.J.; Baek, J.H.; Lee, C.H.; Yu, Y.G.; Nam, H.W. Comprehensive proteome analysis of the excretory/secretory proteins of toxoplasma gondii. Bull. Korean Chem. Soc. 2014, 35, 3071–3076. [Google Scholar] [CrossRef] [Green Version]
- Barylyuk, K.; Koreny, L.; Ke, H.; Butterworth, S.; Crook, O.M.; Lassadi, I.; Gupta, V.; Tromer, E.; Mourier, T.; Stevens, T.J.; et al. A subcellular atlas of Toxoplasma reveals the functional context of the proteome. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Christoforou, A.; Mulvey, C.M.; Breckels, L.M.; Geladaki, A.; Hurrell, T.; Hayward, P.C.; Naake, T.; Gatto, L.; Viner, R.; Arias, A.M.; et al. A draft map of the mouse pluripotent stem cell spatial proteome. Nat. Commun. 2016, 7, 9992. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, C.M.; Breckels, L.M.; Geladaki, A.; Britovšek, N.K.; Nightingale, D.J.H.; Christoforou, A.; Elzek, M.; Deery, M.J.; Gatto, L.; Lilley, K.S. Using hyperLOPIT to perform high-resolution mapping of the spatial proteome. Nat. Protoc. 2017, 12, 1110–1135. [Google Scholar] [CrossRef]
- Pozio, E.; Darwin Murrell, K. Systematics and Epidemiology of Trichinella. Adv. Parasitol. 2006, 63, 367–439. [Google Scholar]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Zhang, N.Z.; Li, W.H.; Li, L.; Yan, H.B.; Qu, Z.G.; Li, T.T.; Cui, J.M.; Yang, Y.; Jia, W.Z.; et al. Proteomic analysis of differentially expressed proteins in the three developmental stages of Trichinella spiralis. Vet. Parasitol. 2016, 231, 32–38. [Google Scholar] [CrossRef]
- Ren, H.N.; Liu, R.D.; Song, Y.Y.; Zhuo, T.X.; Guo, K.X.; Zhang, Y.; Jiang, P.; Wang, Z.Q.; Cui, J. Label-free quantitative proteomic analysis of molting-related proteins of Trichinella spiralis intestinal infective larvae. Vet. Res. 2019, 50, 70. [Google Scholar] [CrossRef] [Green Version]
- Grzelak, S.; Moskwa, B.; Bień, J. Trichinella britovi muscle larvae and adult worms: Stage-specific and common antigens detected by two-dimensional gel electrophoresis-based immunoblotting 06 Biological Sciences 0601 Biochemistry and Cell Biology. Parasites Vectors 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dea-Ayuela, M.A.; Bolás-Fernández, F. Two-dimensional electrophoresis and mass spectrometry for the identification of species-specific Trichinella antigens. Vet. Parasitol. 2005, 132, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, W.; Sun, X.; Zhao, X.; Yuan, G.; Sun, Q.; Huang, J.; Zhu, X. Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasites Vectors 2015, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somboonpatarakun, C.; Rodpai, R.; Intapan, P.M.; Sanpool, O.; Sadaow, L.; Wongkham, C.; Insawang, T.; Boonmars, T.; Maleewong, W. Immuno-proteomic analysis of Trichinella spiralis, T. pseudospiralis, and T. papuae extracts recognized by human T. spiralis-infected sera. Parasitol. Res. 2018, 117, 201–212. [Google Scholar] [CrossRef]
- Liu, R.D.; Cui, J.; Wang, L.; Al, E. Identification of surface proteins of Trichinella spiralis muscle larvae using immunoproteomics. Trop. Biomed. 2014, 31, 579–591. [Google Scholar]
- Liu, R.D.; Cui, J.; Liu, X.L.; Jiang, P.; Sun, G.G.; Zhang, X.; Long, S.R.; Wang, L.; Wang, Z.Q. Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop. 2015, 150, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, X.; Zhu, H.; Wang, X.; Shi, H.; Tang, B.; Boireau, P.; Cai, X.; Luo, X.; Liu, M.; et al. Immunoproteomic analysis of the excretory-secretory products of Trichinella pseudospiralis adult worms and newborn larvae. Parasites Vectors 2017, 10, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzelak, S.; Stachyra, A.; Bień-Kalinowska, J. The first analysis of Trichinella spiralis and Trichinella britovi adult worm excretory-secretory proteins by two-dimensional electrophoresis coupled with LC-MS/MS. Vet. Parasitol. 2020, 109096. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X.; Tang, B.; Zhang, Y.; Zhang, L.; Cai, X.; Lin, J.; Jia, W.; Boireau, P.; Liu, M.; et al. Comparative analysis of excretory–secretory products of muscle larvae of three isolates of Trichinella pseudospiralis by the iTRAQ method. Vet. Parasitol. 2020, 109119. [Google Scholar] [CrossRef]
- Djurković-Djaković, O.; Bobić, B.; Nikolić, A.; Klun, I.; Dupouy-Camet, J. Pork as a source of human parasitic infection. Clin. Microbiol. Infect. 2013, 19, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Dorny, P.; Vallée, I.; Alban, L.; Boes, J.; Boireau, P.; Boué, F.; Claes, M.; Cook, A.J.C.; Enemark, H.; van der Giessen, J.; et al. Development of harmonised schemes for the monitoring and reporting of Cysticercus in animals and foodstuffs in the European Union. EFSA Support. Publ. 2010, 7. [Google Scholar] [CrossRef] [Green Version]
- García, H.H.; Gonzalez, A.E.; Evans, C.A.; Gilman, R.H. Taenia solium cysticercosis. Lancet 2003, 362, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Xiao, L.L.; Bao, H.E.; Mu, R. Total protein analysis by two-dimensional electrophoresis in cysticerci of Taenia solium and Taenia asiatica. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2011, 29, 188–190. [Google Scholar] [PubMed]
- Santivañez, S.J.; Hernández-González, A.; Chile, N.; Oleaga, A.; Arana, Y.; Palma, S.; Verastegui, M.; Gonzalez, A.E.; Gilman, R.; Garcia, H.H.; et al. Proteomic study of activated Taenia solium oncospheres. Mol. Biochem. Parasitol. 2010, 171, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Masmela, Y.; Fragoso, G.; Ambrosio, J.R.; Mendoza-Hernández, G.; Rosas, G.; Estrada, K.; Carrero, J.C.; Sciutto, E.; Laclette, J.P.; Bobes, R.J. Immunodiagnosis of porcine cysticercosis: Identification of candidate antigens through immunoproteomics. Vet. J. 2013, 198, 656–660. [Google Scholar] [CrossRef]
- Esquivel-Velázquez, M.; Larralde, C.; Morales, J.; Ostoa-Saloma, P. Protein and antigen diversity in the vesicular fluid of taenia solium cysticerci dissected from naturally infected pigs. Int. J. Biol. Sci. 2011, 7, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Perea, J.; Moguel, B.; Mendoza-Hernández, G.; Fragoso, G.; Sciutto, E.; Bobes, R.J.; Laclette, J.P. Identification and quantification of host proteins in the vesicular fluid of porcine Taenia solium cysticerci. Exp. Parasitol. 2014, 143, 11–17. [Google Scholar] [CrossRef]
- Bae, Y.A.; Yeom, J.S.; Wang, H.; Kim, S.H.; Ahn, C.S.; Kim, J.T.; Yang, H.J.; Kong, Y. Taenia solium metacestode fasciclin-like protein is reactive with sera of chronic neurocysticercosis. Trop. Med. Int. Health 2014, 19, 719–725. [Google Scholar] [CrossRef]
- Navarrete-Perea, J.; Moguel, B.; Bobes, R.J.; Villalobos, N.; Carrero, J.C.; Sciutto, E.; Soberón, X.; Laclette, J.P. Protein profiles of Taenia solium cysts obtained from skeletal muscles and the central nervous system of pigs: Search for tissue-specific proteins. Exp. Parasitol. 2017, 172, 23–29. [Google Scholar] [CrossRef]
- Navarrete-Perea, J.; Isasa, M.; Paulo, J.A.; Corral-Corral, R.; Flores-Bautista, J.; Hernández-Téllez, B.; Bobes, R.J.; Fragoso, G.; Sciutto, E.; Soberón, X.; et al. Quantitative multiplexed proteomics of Taenia solium cysts obtained from the skeletal muscle and central nervous system of pigs. PLoS Negl. Trop. Dis. 2017, 11, e0005962. [Google Scholar] [CrossRef] [Green Version]
- da Costa, G.C.V.; Peralta, R.H.S.; Kalume, D.E.; Alves, A.L.G.M.; Peralta, J.M. A gel-free proteomic analysis of Taenia solium and Taenia crassiceps cysticerci vesicular extracts. Parasitol. Res. 2018, 117, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- Victor, B.; Kanobana, K.; Gabriël, S.; Polman, K.; Deckers, N.; Dorny, P.; Deelder, A.M.; Palmblad, M. Proteomic analysis of Taenia solium metacestode excretion-secretion proteins. Proteomics 2012, 12, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R. Sarcocystis spp. in Human Infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayer, R.; Heydorn, A.O.; Johnson, A.J.; Leek, R.G. Transmission of Sarcocystis suihominis from humans to swine to nonhuman primates (Pan troglodytes, Macaca mulatta, Macaca irus). Z. Parasitenkd. Parasitol. Res. 1979, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.H.; Sithithaworn, P.; Petney, T.N. Opisthorchis viverrini: An underestimated parasite in world health. Trends Parasitol. 2008, 24, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Traub, R.J.; Macaranas, J.; Mungthin, M.; Leelayoova, S.; Cribb, T.; Murrell, K.D.; Thompson, R.C.A. A New PCR-Based Approach Indicates the Range of Clonorchis sinensis Now Extends to Central Thailand. PLoS Negl. Trop. Dis. 2009, 3, e367. [Google Scholar] [CrossRef] [Green Version]
- Wykoff, D.E.; Harinasuta, C.; Juttijudata, P.; Winn, M.M. Opisthorchis viverrini in Thailand: The Life Cycle and Comparison with O. felineus. J. Parasitol. 1965, 51, 207. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef]
- Prasopdee, S.; Thitapakorn, V.; Sathavornmanee, T.; Tesana, S. A comprehensive review of omics and host-parasite interplays studies, towards control of Opisthorchis viverrini infection for prevention of cholangiocarcinoma. Acta Trop. 2019, 196, 76–82. [Google Scholar] [CrossRef]
- Boonmee, S.; Imtawil, K.; Wongkham, C.; Wongkham, S. Comparative proteomic analysis of juvenile and adult liver fluke, Opisthorchis viverrini. Acta Trop. 2003, 88, 233–238. [Google Scholar] [CrossRef]
- Mulvenna, J.; Sripa, B.; Brindley, P.J.; Gorman, J.; Jones, M.K.; Colgrave, M.L.; Jones, A.; Nawaratna, S.; Laha, T.; Suttiprapa, S.; et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics 2010, 10, 1063–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasopdee, S.; Tesana, S.; Cantacessi, C.; Laha, T.; Mulvenna, J.; Grams, R.; Loukas, A.; Sotillo, J. Proteomic profile of Bithynia siamensis goniomphalos snails upon infection with the carcinogenic liver fluke Opisthorchis viverrini. J. Proteom. 2015, 113, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwannatrai, K.; Suwannatrai, A.; Tabsripair, P.; Welbat, J.U.; Tangkawattana, S.; Cantacessi, C.; Mulvenna, J.; Tesana, S.; Loukas, A.; Sotillo, J. Differential Protein Expression in the Hemolymph of Bithynia siamensis goniomphalos Infected with Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2016, 10, e0005104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.P.; Lai, D.H.; Zhu, X.Q.; Chen, X.G.; Lun, Z.R. Human angiostrongyliasis. Lancet Infect. Dis. 2008, 8, 621–630. [Google Scholar] [CrossRef]
- Martins, Y.C.; Tanowitz, H.B.; Kazacos, K.R. Central nervous system manifestations of Angiostrongylus cantonensis infection. Acta Trop. 2015, 141PA, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Sawanyawisuth, K.; Kitthaweesin, K.; Limpawattana, P.; Intapan, P.M.; Tiamkao, S.; Jitpimolmard, S.; Chotmongkol, V. Intraocular angiostrongyliasis: Clinical findings, treatments and outcomes. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 497–501. [Google Scholar] [CrossRef]
- Mattis, A.; Mowatt, L.; Lue, A.; Lindo, J.; Vaughan, H. Ocular Angiostrongyliasis—First case report from Jamaica. West Indian Med. J. 2009, 58, 383–385. [Google Scholar]
- Chen, K.Y.; Cheng, C.J.; Yen, C.M.; Tang, P.; Wang, L.C. Comparative studies on the proteomic expression patterns in the third- and fifth-stage larvae of Angiostrongylus cantonensis. Parasitol. Res. 2014, 113, 3591–3600. [Google Scholar] [CrossRef]
- Huang, H.C.; Yao, L.L.; Song, Z.M.; Li, X.P.; Hua, Q.Q.; Li, Q.; Pan, C.W.; Xia, C.M. Development-Specific Differences in the Proteomics of Angiostrongylus cantonensis. PLoS ONE 2013, 8, e76982. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.Y.; Lu, P.J.; Cheng, C.J.; Jhan, K.Y.; Yeh, S.C.; Wang, L.C. Proteomic analysis of excretory-secretory products from the young adults of Angiostrongylus cantonensis. Mem. Inst. Oswaldo Cruz 2019, 114, e180556. [Google Scholar] [CrossRef]
- Mega, J.D.; Galdos-Cardenas, G.; Gilman, R.H. Tapeworm Infections. In Hunter’s Tropical Medicine and Emerging Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 895–902. [Google Scholar]
- Scholz, T.; Garcia, H.H.; Kuchta, R.; Wicht, B. Update on the Human Broad Tapeworm (Genus Diphyllobothrium), Including Clinical Relevance. Clin. Microbiol. Rev. 2009, 22, 146–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, D.; Agatsuma, T.; Wang, W. Paragonimiasis. In Food-Borne Parasitic Zoonoses Fish and Plant-Borne Parasites; Springer: New York, NY, USA, 2007; pp. 117–150. [Google Scholar]
- Lee, E.G.; Na, B.K.; Bae, Y.A.; Kim, S.H.; Je, E.Y.; Ju, J.W.; Cho, S.H.; Kim, T.S.; Kang, S.Y.; Cho, S.Y.; et al. Identification of immunodominant excretory–secretory cysteine proteases of adult Paragonimus westermani by proteome analysis. Proteomics 2006, 6, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, S.I.; Hong, K.M.; Kim, M.J.; Shin, C.H.; Ryu, J.S.; Min, D.Y.; Lee, J.B.; Hwang, U.W. Characterization and classification of five cysteine proteinases expressed by Paragonimus westermani adult worm. Exp. Parasitol. 2002, 102, 143–149. [Google Scholar] [CrossRef]
- Chai, J.Y. Human Intestinal Flukes; Springer: Dordrecht, The Netherlands, 2019; ISBN 978-94-024-1702-9. [Google Scholar]
- Fried, B.; Graczyk, T.K.; Tamang, L. Food-borne intestinal trematodiases in humans. Parasitol. Res. 2004, 93, 159–170. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Shah, R.M.; Karpe, A.V.; Morrison, P.D.; Kouremenos, K.; Beale, D.J.; Palombo, E.A. Detection of foodborne pathogens using proteomics and metabolomics-based approaches. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Rousseau, A.; La Carbona, S.; Dumètre, A.; Robertson, L.J.; Gargala, G.; Escotte-Binet, S.; Favennec, L.; Villena, I.; Gérard, C.; Aubert, D. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: A review of methods. Parasite 2018, 25, 14. [Google Scholar] [CrossRef] [Green Version]
- Gamble, H.R.; Murrell, K.D. Detection of parasites in food. Parasitology 1999, 117, 97–111. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and their applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [Green Version]
- Jagadeesh, D.S.; Kannegundla, U.; Reddy, R.K. Application of proteomic tools in food quality and safety. Adv. Anim. Vet. Sci. 2017, 5, 213–225. [Google Scholar] [CrossRef]
- Bassols, A.; Turk, R.; Roncada, P. A Proteomics Perspective: From Animal Welfare to Food Safety. Curr. Protein Pept. Sci. 2014, 15, 156–168. [Google Scholar] [CrossRef]
- Borràs, E.; Sabidó, E. What is targeted proteomics? A concise revision of targeted acquisition and targeted data analysis in mass spectrometry. Proteomics 2017, 17, 1700180. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Abel, P.M.; Agranoff, D.; Stich, A.; Tarelli, E.; Bell, B.A.; Planche, T.; Loosemore, A.; Saadoun, S.; Wilkins, P.; et al. A novel and accurate diagnostic test for human African trypanosomiasis. Lancet 2004, 363, 1358–1363. [Google Scholar] [CrossRef]
- Rioux, M.C.; Carmona, C.; Acosta, D.; Ward, B.; Ndao, M.; Gibbs, B.F.; Bennett, H.P.; Spithill, T.W. Discovery and validation of serum biomarkers expressed over the first twelve weeks of Fasciola hepatica infection in sheep. Int. J. Parasitol. 2008, 38, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Deckers, N.; Dorny, P.; Kanobana, K.; Vercruysse, J.; Gonzalez, A.E.; Ward, B.; Ndao, M. Use of ProteinChip technology for identifying biomarkers of parasitic diseases: The example of porcine cysticercosis (Taenia solium). Exp. Parasitol. 2008, 120, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, C.; Chatelain, E.; Jackson, Y.; Miao, Q.; Ward, B.J.; Chappuis, F.; Ndao, M. Serum biomarkers predictive of cure in Chagas disease patients after nifurtimox treatment. BMC Infect. Dis. 2014, 14, 302. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Ovejero, C.; Benito-Lopez, F.; Díez, P.; Casulli, A.; Siles-Lucas, M.; Fuentes, M.; Manzano-Román, R. Sensing parasites: Proteomic and advanced bio-detection alternatives. J. Proteom. 2016, 136, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Klimpel, S.; Palm, H.W. Anisakid Nematode (Ascaridoidea) Life Cycles and Distribution: Increasing Zoonotic Potential in the Time of Climate Change? In Progress in Parasitology. Parasitology Research Monographs; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–222. ISBN 978-3-642-21395-3. [Google Scholar]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From Obscure Infectious Worm to Inducer of Immune Hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [Green Version]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish- from infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: Dangerous—Dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- D’Amelio, S.; Lombardo, F.; Pizzarelli, A.; Bellini, I.; Cavallero, S. Advances in Omic Studies Drive Discoveries in the Biology of Anisakid Nematodes. Genes 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Fæste, C.K.; Jonscher, K.R.; Dooper, M.M.W.B.; Egge-Jacobsen, W.; Moen, A.; Daschner, A.; Egaas, E.; Christians, U. Characterisation of potential novel allergens in the fish parasite Anisakis simplex. EuPA Open Proteom. 2014, 4, 140–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, I.; Łopieńska-Biernat, E.; Stryiński, R.; Mateos, J.; Carrera, M. Comparative proteomics analysis of Anisakis simplex s.s.—Evaluation of the response of invasive larvae to ivermectin. Genes 2020, 11, 11060710. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mahillo, A.I.; González-Muñoz, M.; de las Heras, C.; Tejada, M.; Moneo, I. Quantification of Anisakis simplex Allergens in Fresh, Long-Term Frozen, and Cooked Fish Muscle. Foodborne Pathog. Dis. 2010, 7, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fæste, C.K.; Plassen, C.; Løvberg, K.E.; Moen, A.; Egaas, E. Detection of Proteins from the Fish Parasite Anisakis simplex in Norwegian Farmed Salmon and Processed Fish Products. Food Anal. Methods 2015, 8, 1390–1402. [Google Scholar] [CrossRef]
- Fæste, C.K.; Moen, A.; Schniedewind, B.; Haug Anonsen, J.; Klawitter, J.; Christians, U. Development of liquid chromatography-tandem mass spectrometry methods for the quantitation of Anisakis simplex proteins in fish. J. Chromatogr. A 2016, 1432, 58–72. [Google Scholar] [CrossRef]
- Carrera, M.; Gallardo, J.M.; Pascual, S.; González, Á.F.; Medina, I. Protein biomarker discovery and fast monitoring for the identification and detection of Anisakids by parallel reaction monitoring (PRM) mass spectrometry. J. Proteom. 2016, 142, 130–137. [Google Scholar] [CrossRef] [Green Version]
| Rank | Foodborne Parasites | Infective Life Stage | Transmission Route |
|---|---|---|---|
| 1 | Echinococcus multiocularis | Eggs | soilborne |
| 2 | Toxoplasma gondii | Fecal oocyst or tissue cyst (bradyzoites) | soil- and meatborne |
| 3 | Trichinella spiralis | Larvae in a nurse cell | meatborne |
| 4 | Echinococcus granulosus | Eggs | soilborne |
| 5 | Cryptosporidium spp. | Oocysts | waterborne |
| 6 | Trichinella spp. other than T. spiralis | Larvae | meatborne |
| 7 | Anisakidae | Larvae | seafood-borne |
| 8 | Giardia lamblia | Cysts | waterborne |
| 9 | Toxocara spp. | Eggs | soilborne |
| 10 | Taenia solium | Eggs/Cysticerci | meatborne |
| 11 | Opisthorchiidae | Metacercariae | seafood-borne |
| 12 | Ascaris spp. | Fertilized eggs | soilborne |
| 13 | Angiostrongylus cantonesis | Larvae | seafood-borne |
| 14 | Entamoeba histolytica | Cysts | waterborne |
| 15 | Taenia saginata | Eggs/Cysticerci | meatborne |
| 16 | Diphyllobothrium spp. | Plerocercoid larvae | seafood-borne |
| 17 | Fasciola spp. | Metacercariae | plantborne |
| 18 | Sarcocystis spp. | Cysts with bradyzoites | meatborne |
| 19 | Trypanosoma cruzi | Metacyclic trypomastigotes | soilborne |
| 20 | Balantidium coli | Cysts | soil- and waterborne |
| 21 | Cyclospora cayetanensis | Sporulated oocysts | waterborne |
| 22 | Trichuris trichiura | Eggs | soilborne |
| 23 | Paragonimus spp. | Metacercariae | seafood-borne |
| 24 | Heterophyidae | Metacercariae | seafood-borne |
| 25 | Spirometra spp. | Pro-/Plerocercoid larvae | water- and meatborne |
| Systematics | Species/Caused Disease | Common Name/Human Organ Where Occures | |||
|---|---|---|---|---|---|
| Flatworm infection | Platyhelminthes * | Rhabditophora ^ | Trematoda ” Plagiorchiida ’ | Fasciola hepatica/ F. gigantica Fasciolosis | Common liver fluke; liver |
| Opisthorchis viverrini/ O. felineus Opisthorchiasis | Southeast Asian/Cat liver fluke; liver | ||||
| Paragonimus westermani Paragonimiasis | Oriental lung fluke; lung | ||||
| Heterophyidae Heterophyiasis | - ; small intestine | ||||
| Cestoda ^ | Cyclophyllidea ’ | Echinococcus granulosus/ E. multilocularis Echinococcosis | Dog tapeworm/Hydatid worm; liver and other organs | ||
| Taenia saginata/T. solium Taeniasis/Cysticercosis | Beef/Pork tapeworm; small intestine | ||||
| Pseudophyllidea ’ | Diphyllobothrium latum Diphyllobothriasis | Broad fish tapeworm; small intestine | |||
| Spirometra spp. Sparganosis | - ; subcutaneous tissues or muscle | ||||
| Roundworm infection | Nematoda * | Chromadorea ^ | Rhabditidia ’ | Angiostrongylus cantonensis Angiostrongyliasis | Rat lungworm; brain and nervous system |
| Ascaridida ’ | Ascaris lumbricoides Ascariasis | Large roundworm; small intestine | |||
| Anisakis simplex s.s. /A. pegreffii Anisakiasis | Herring worm; gastrointestinal tract | ||||
| Toxocara canis/T. cati Visceral larva migrans/Toxocariasis | Dog/feline roundworm; eye, liver, lungs etc. | ||||
| Enoplea ^ | Trichocephalida ’ | Trichinella spiralis Trichinosis | Trichna worm; intestine, muscle and sometimes other organs | ||
| Trichuris trichiura Trichuriasis | Whipworm; large intestine | ||||
| Protozoan infection | Apicomplexa * | Eucoccidiorida ’ | Toxoplasma gondii Toxoplasmosis | - ; brain, eye, lungs, heart, muscle etc. | |
| Cryptosporidium parvum Cryptosporidiosis | - ; intestinal tract | ||||
| Sarcocystis spp. Sarcocystosis | - ; blood vessels, muscles, intestine | ||||
| Cyclospora cayetanensis Cyclosporiasis | - ; stomach, small intestine | ||||
| Metamonada * | Diplomonadida ’ | Giardia lamblia Giardiasis | - ; small intestine | ||
| Amoebozoa * | Amoebida ’ | Entamoeba histolytica Amoebiasis | - ; large intestine and other organs | ||
| Euglenozoa * | Kinetoplastida ’ | Trypanosoma cruzi Chagas disease | - ; heart, oesophagus, colon, nervous system | ||
| Ciliophora * | Heterotrichida ’ | Balantidium coli Balantidiasis | - ; cecum and colon | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stryiński, R.; Łopieńska-Biernat, E.; Carrera, M. Proteomic Insights into the Biology of the Most Important Foodborne Parasites in Europe. Foods 2020, 9, 1403. https://doi.org/10.3390/foods9101403
Stryiński R, Łopieńska-Biernat E, Carrera M. Proteomic Insights into the Biology of the Most Important Foodborne Parasites in Europe. Foods. 2020; 9(10):1403. https://doi.org/10.3390/foods9101403
Chicago/Turabian StyleStryiński, Robert, Elżbieta Łopieńska-Biernat, and Mónica Carrera. 2020. "Proteomic Insights into the Biology of the Most Important Foodborne Parasites in Europe" Foods 9, no. 10: 1403. https://doi.org/10.3390/foods9101403
APA StyleStryiński, R., Łopieńska-Biernat, E., & Carrera, M. (2020). Proteomic Insights into the Biology of the Most Important Foodborne Parasites in Europe. Foods, 9(10), 1403. https://doi.org/10.3390/foods9101403






