Formulation and Preparation of Water-In-Oil-In-Water Emulsions Loaded with a Phenolic-Rich Inner Aqueous Phase by Application of High Energy Emulsification Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Phenolic Rich Olive Mill Aqueous Phase
2.3. Preparation of Primary Emulsion (W/O)
2.4. Preparation of Double Emulsion (W/O/W)
2.5. Droplet Size Analysis of the Emulsions
2.6. ζ-Potential
2.7. Total Phenolic Content
2.8. Antioxidant Activity
2.9. Chemical Stability Measurements
2.10. Physical Stability Measurements
2.11. Experimental Design
3. Results and Discussion
3.1. Determination of W/O/W Double Emulsion Formulation
3.1.1. Model Fitting of Formulation
3.1.2. Response Surface Analysis
3.1.3. Optimization of Double Emulsion Formulation
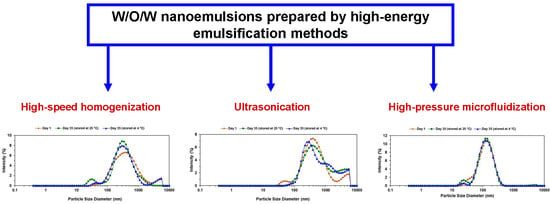
3.2. Effect of the High Energy Emulsification Method on Double Emulsion Droplet Size
3.2.1. Model Fitting of Emulsification Method
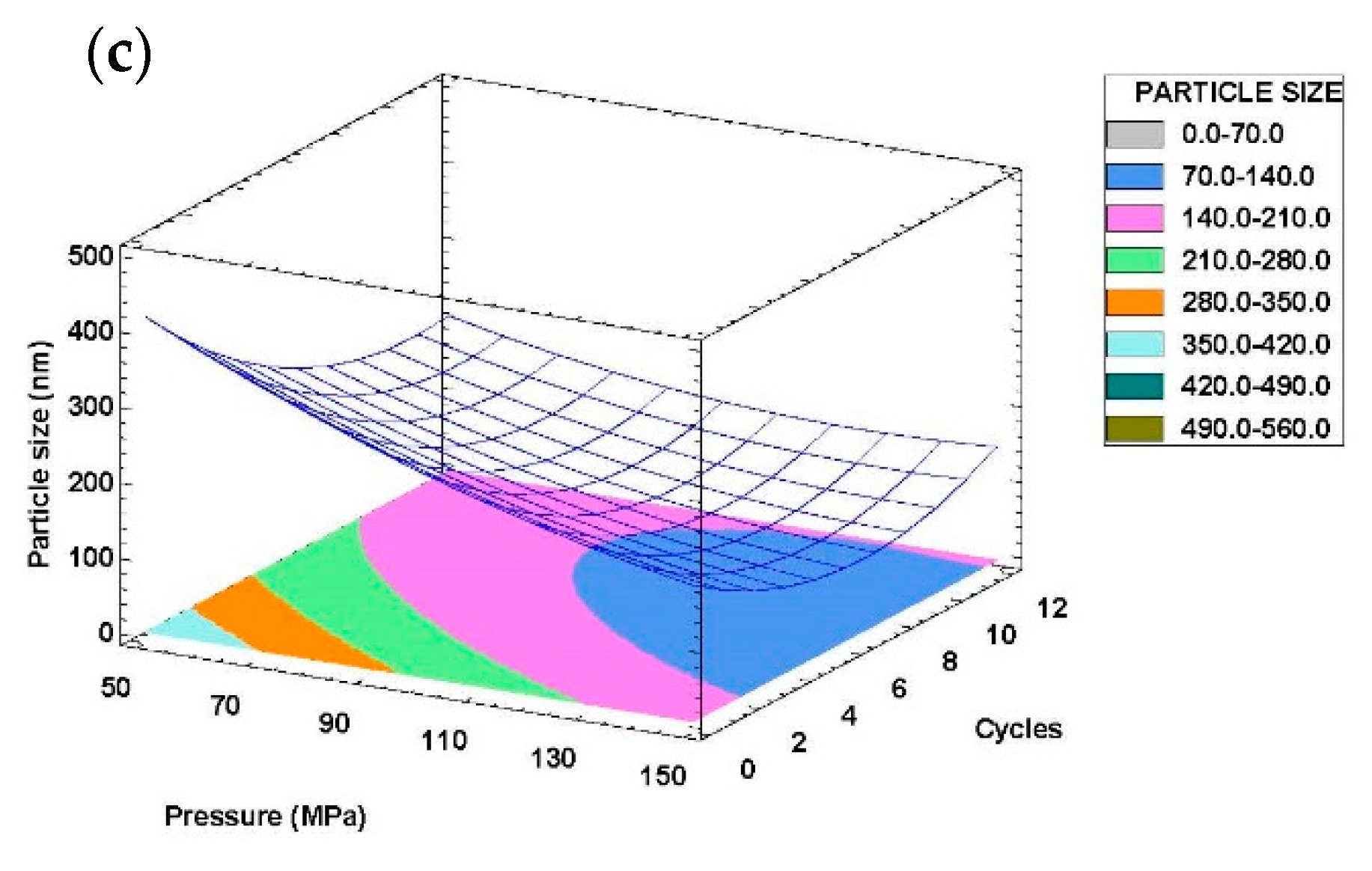
3.2.2. Response Surface Analysis
3.2.3. Optimization of W/O/W Emulsion Preparation Conditions
3.3. Effect of the High Energy Emulsification Method on Double Emulsion Droplet Size
3.3.1. Stability Evaluations During Storage
3.3.2. Retention Properties of Nanoemulsions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friberg, S.; Larsson, K.; Sjöblom, J. Food Emulsions, 4th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Bénichou, A.; Aserin, A.; Garti, N. Double emulsions stabilized with hybrids of natural polymers for entrapment and slow release of active matters. Adv. Colloid Interface Sci. 2004, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Van Der Graaf, S.; Schroën, K.; Boom, R. Preparation of double emulsions by membrane emulsification—a review. J. Membr. Sci. 2005, 251, 7–15. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols – a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Anton, H.; Gayet, P.; Benoit, J.-P.; Saulnier, P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef]
- Anton, H.; Vandamme, T.F. The universality of low-energy nano-emulsification. Int. J. Pharm. 2009, 377, 142–147. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 303–318. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of Oil Type on Nanoemulsion Formation and Ostwald Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Llinares, R.; Santos, J.; Trujillo-Cayado, L.A.; Ramírez, P.; Muñoz, J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT 2018, 97, 370–375. [Google Scholar] [CrossRef]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushani, J.A.; Kurrey, N.K.; Anandharamakrishnan, C. Nanoencapsulation of green tea catechins by electrospraying technique and its effect on controlled release and in-vitro permeability. J. Food Eng. 2017, 199, 82–92. [Google Scholar] [CrossRef]
- Alves, A.D.C.S.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Pittia, P.; Di Mattia, C.D. Effect of phenolic antioxidants on the dispersion state and chemical stability of olive oil O/W emulsions. Food Res. Int. 2009, 42, 1163–1170. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Shashidhar, M.; Balaraman, M. Delivery of green tea catechins through Oil-in-Water (O/W) nanoemulsion and assessment of storage stability. J. Food Eng. 2017, 199, 65–76. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 69–79. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.; Mann, B.; Kumar, R.; Singh, R.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tuyishime, M.A.; Matabaro, E.; Habinshuti, I.; Hitabatuma, A.; Korma, S.A.; Ali, A.H. Green tea polyphenols-proteins nanocomplexes foaming behavior, nanoemulsions and their applications. IJAIR 2016, 5, 149–156. [Google Scholar]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Estévez, M.; Güell, C.; De Lamo-Castellví, S.; Ferrando, M. Encapsulation of grape seed phenolic-rich extract within W/O/W emulsions stabilized with complexed biopolymers: Evaluation of their stability and release. Food Chem. 2019, 272, 478–487. [Google Scholar] [CrossRef]
- Ye, Q.; Li, T.; Li, J.; Liu, L.; Dou, X.; Zhang, X. Development and evaluation of tea polyphenols loaded water in oil emulsion with zein as stabilizer. J. Drug Deliv. Sci. Technol. 2020, 56, 101528. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Garti, N.; Bisperink, C. Double emulsions: Progress and applications. Curr. Opin. Colloid Interface Sci. 1998, 3, 657–667. [Google Scholar] [CrossRef]
- Cournarie, F.; Savelli, M.-P.; Rosilio, V.; Bretez, F.; Vauthier, C.; Grossiord, J.-L.; Seiller, M. Insulin-loaded W/O/W multiple emulsions: Comparison of the performances of systems prepared with medium-chain-triglycerides and fish oil. Eur. J. Pharm. Biopharm. 2004, 58, 477–482. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Li, Y.; Le Maux, S.; Xiao, H.; McClements, D.J. Emulsion-Based Delivery Systems for Tributyrin, a Potential Colon Cancer Preventative Agent. J. Agric. Food Chem. 2009, 57, 9243–9249. [Google Scholar] [CrossRef]
- Mun, S.; McClements, D.J. Influence of Interfacial Characteristics on Ostwald Ripening in Hydrocarbon Oil-in-Water Emulsions. Langmuir 2006, 22, 1551–1554. [Google Scholar] [CrossRef]
- Mao, L.; Xu, D.; Yang, J.; Yuan, F.; Gao, Y.; Zhao, J. Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Bazzarelli, F.; Poerio, T.; Mazzei, R.; D’Agostino, N.; Giorno, L. Study of OMWWs suspended solids destabilization to improve membrane processes performance. Sep. Purif. Technol. 2015, 149, 183–189. [Google Scholar] [CrossRef]
- Hoff, J.E.; Singleton, K.I. A method for determination of tannins in foods by means of immobilized protein. J. Food Sci. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.O.; Mulvihill, D.M. Water soluble inner aqueous phase markers as indicators of the encapsulation properties of water-in-oil-in-water emulsions stabilized with sodium caseinate. Food Hydrocoll. 2009, 23, 2339–2345. [Google Scholar] [CrossRef]
- Allende, D.; Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J. Destabilization-Enhanced Centrifugation of Metalworking Oil-in-Water Emulsions: Effect of Demulsifying Agents. Chem. Eng. Technol. 2008, 31, 1007–1014. [Google Scholar] [CrossRef]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltrán, S.; Escudero, I.; González-Sanjosé, M.L. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Ghorbannezhad, P.; Bay, A.; Yolmeh, M.; Yadollahi, R.; Moghadam, J.Y. Optimization of coagulation–flocculation process for medium density fiberboard (MDF) wastewater through response surface methodology. Desalin. Water Treat. 2016, 57, 26916–26931. [Google Scholar] [CrossRef]
- Hu, Y.-T.; Ting, Y.; Hu, J.-Y.; Hsieh, S.-L. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Karthivashan, G.; Park, S.Y.; Kim, J.; Choi, D.-K. Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments. Int. J. Nanomed. 2018, 13, 6109–6121. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; He, Y.; Bhandari, B. Nano-Emulsion Production by Sonication and Microfluidization—A Comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Kentish, S.; Wooster, T.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Khadem, B.; Sheibat-Othman, N. Theoretical and Experimental Investigations of Double Emulsion Preparation by Ultrasonication. Ind. Eng. Chem. Res. 2019, 58, 8220–8230. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, V. Ultrasonication assisted formation and stability of water-in-oil nanoemulsions: Optimization and ternary diagram analysis. Ultrason. Sonochem. 2018, 49, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Chiang, B.-H. Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of olive oil based O/W nanoemulsions prepared through ultrasonic homogenization: A response surface methodology approach. Food Chem. 2017, 229, 790–796. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Schuch, A.; Wrenger, J.; Karbstein, H.P. Production of W/O/W double emulsions. Part II: Influence of emulsification device on release of water by coalescence. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 344–351. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Kato, E.T.M.; De Araujo, G.L.B.; Löbenberg, R.; Monteiro, L.M.; Lourenço, F.R.; Bou-Chacra, N.A. Olive oil nanoemulsion preparation using high-pressure homogenization and d-phase emulsification—A design space approach. J. Drug Deliv. Sci. Technol. 2019, 49, 622–631. [Google Scholar] [CrossRef]
- Leong, T.; Wooster, T.; Kentish, S.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Chen, L.-W. Comparison of fuel properties and emission characteristics of two- and three-phase emulsions prepared by ultrasonically vibrating and mechanically homogenizing emulsification methods. Fuel 2008, 87, 2154–2161. [Google Scholar] [CrossRef]
- Đorđević, V.; Belščak-Cvitanović, A.; Drvenica, I.; Komes, D.; Nedovic, V.; Bugarski, B.M. Nanoscale nutrient delivery systems. In Nutrient Delivery. Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2017; Volume 5, pp. 87–139. [Google Scholar]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Atiemo-Obeng, V.A.; Calabrese, R.V. Rotor-stator mixing devices. In Handbook of Industrial Mixing: Science and Practice, 1st ed.; Paul, E.L., Atiemo-Obeng, V.A., Kresta, S.M., Eds.; Wiley: Hoboken, NJ, USA, 2003; pp. 479–505. [Google Scholar]
- Mortensen, H.H.; Innings, F.; Håkansson, A. The effect of stator design on flowrate and velocity fields in a rotor-stator mixer—An experimental investigation. Chem. Eng. Res. Des. 2017, 121, 245–254. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, S.; Li, W. High shear mixers: A review of typical applications and studies on power draw, flow pattern, energy dissipation and transfer properties. Chem. Eng. Process. Process. Intensif. 2012, 57, 25–41. [Google Scholar] [CrossRef]
- Anarjan, N.; Mirhosseini, H.; Baharin, B.S.; Tan, C.P. Effect of processing conditions on physicochemical properties of astaxanthin nanodispersions. Food Chem. 2010, 123, 477–483. [Google Scholar] [CrossRef]
- Gaikwad, S.G.; Pandit, A.B. Ultrasound emulsification: Effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason. Sonochem. 2008, 15, 554–563. [Google Scholar] [CrossRef]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Weiss, M.; Kunzek, H. Influence of the emulsion components and preparation method on the laboratory-scale preparation of o/w emulsions containing different types of dispersed phases and/or emulsifiers. Food/Nahrung 2002, 46, 294–301. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Sriamornsak, P. Effect of pH on Stability of Oil-in-Water Emulsions Stabilized by Pectin-Zein Complexes. Adv. Mater. Res. 2013, 747, 127–130. [Google Scholar] [CrossRef]
- Akhtar, M.; Murray, B.S.; Afeisume, E.I.; Khew, S.H. Encapsulation of flavonoid in multiple emulsion using spinning disc reactor technology. Food Hydrocoll. 2014, 34, 62–67. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Perrechil, F.D.A.; Cunha, R.L. Role of the phases composition on the incorporation of gallic acid in O/W and W/O emulsions. J. Food Eng. 2016, 168, 205–214. [Google Scholar] [CrossRef] [Green Version]






| Run | Independent Variables | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Aqueous Phase Content in W/O Emulsion (X1, % w/w) | Surfactant Content in W/O Emulsion (X2, % w/w) | W/O Content in W/O/W Emulsion (X3, % w/w) | Surfactant Content in W/O/W Emulsion (X4, % w/w) | Droplet Size (Y, nm) | Polydispersity Index (PDI) | |||
| Mean | SD | Mean | SD | |||||
| 1 | 12 | 12 | 10 | 7 | 470.4 | 3.7 | 0.426 | 0.043 |
| 2 | 20 | 12 | 25 | 7 | 415.2 | 12.8 | 0.357 | 0.068 |
| 3 | 20 | 4 | 40 | 4 | 512.2 | 14.8 | 0.561 | 0.033 |
| 4 | 20 | 20 | 10 | 4 | 279.7 | 18.1 | 0.439 | 0.015 |
| 5 | 12 | 4 | 25 | 7 | 344.8 | 10.1 | 0.486 | 0.032 |
| 6 | 20 | 4 | 40 | 10 | 1767 | 119.5 | 0.592 | 0.071 |
| 7 | 4 | 20 | 10 | 4 | 388.7 | 6.3 | 0.458 | 0.020 |
| 8 | 20 | 4 | 10 | 10 | 470.3 | 4.7 | 0.437 | 0.024 |
| 9 | 20 | 20 | 40 | 4 | 478.3 | 19.1 | 0.607 | 0.076 |
| 10 | 12 | 12 | 25 | 4 | 316.2 | 13.0 | 0.571 | 0.027 |
| 11 | 4 | 4 | 10 | 10 | 363.5 | 11.4 | 0.329 | 0.054 |
| 12 | 12 | 20 | 25 | 7 | 371.8 | 7.4 | 0.454 | 0.009 |
| 13 | 12 | 12 | 40 | 7 | 768.9 | 17.3 | 0.620 | 0.009 |
| 14 | 20 | 20 | 40 | 10 | 1828 | 58.3 | 0.501 | 0.015 |
| 15 | 12 | 12 | 25 | 7 | 585.8 | 19.1 | 0.553 | 0.041 |
| 16 | 20 | 4 | 10 | 4 | 242.6 | 5.6 | 0.283 | 0.011 |
| 17 | 4 | 20 | 10 | 10 | 330.0 | 10.4 | 0.273 | 0.011 |
| 18 | 4 | 20 | 40 | 4 | 923.6 | 24.3 | 0.610 | 0.045 |
| 19 | 12 | 12 | 25 | 7 | 857.4 | 13.3 | 0.487 | 0.047 |
| 20 | 4 | 4 | 10 | 4 | 232.3 | 2.5 | 0.365 | 0.045 |
| 21 | 20 | 20 | 10 | 10 | 216.3 | 5.0 | 0.460 | 0.032 |
| 22 | 4 | 4 | 40 | 10 | 1336 | 246.6 | 0.907 | 0.162 |
| 23 | 12 | 12 | 25 | 10 | 795.7 | 11.5 | 0.514 | 0.017 |
| 24 | 4 | 20 | 40 | 10 | 362.5 | 8.4 | 0.574 | 0.018 |
| 25 | 4 | 12 | 25 | 7 | 476.5 | 4.2 | 0.467 | 0.006 |
| 26 | 4 | 4 | 40 | 4 | 415.5 | 37.9 | 0.872 | 0.190 |
| Run | Rotor-Stator Mixer | |||||
| Independent Variables | Response Variables | |||||
| Rotation Speed (X1, rpm) | Time (X2, min) | Droplet Size (Y, nm) | Polydispersity Index (PDI) | |||
| Mean | SD | Mean | SD | |||
| 1 | 29000 | 2 | 735.6 | 38.1 | 0.903 | 0.028 |
| 2 | 20000 | 2 | 468.2 | 4.0 | 0.585 | 0.016 |
| 3 | 11000 | 2 | 1255 | 174.6 | 0.392 | 0.147 |
| 4 | 11000 | 6 | 580.0 | 21.9 | 0.487 | 0.032 |
| 5 | 11000 | 10 | 352.9 | 10.5 | 0.554 | 0.076 |
| 6 | 29000 | 6 | 530.4 | 10.2 | 0.484 | 0.029 |
| 7 | 29000 | 10 | 325.5 | 2.6 | 0.539 | 0.007 |
| 8 | 20000 | 10 | 301.7 | 12.1 | 0.439 | 0.030 |
| 9 | 20000 | 6 | 474.5 | 9.7 | 0.457 | 0.032 |
| 10 | 20000 | 6 | 366.4 | 9.5 | 0.551 | 0.019 |
| Run | Ultrasonic Homogenizer | |||||
| Independent Variables | Response Variables | |||||
| Time (X1, min) | Amplitude (X2, %) | Droplet Size (Y, nm) | Polydispersity Index (PDI) | |||
| Mean | SD | Mean | SD | |||
| 11 | 10 | 20 | 338.5 | 16.4 | 0.468 | 0.021 |
| 12 | 6 | 60 | 434.6 | 16.9 | 0.436 | 0.010 |
| 13 | 6 | 20 | 388.1 | 2.4 | 0.468 | 0.013 |
| 14 | 2 | 20 | 622.7 | 26.6 | 0.565 | 0.101 |
| 15 | 6 | 40 | 460.4 | 3.9 | 0.432 | 0.014 |
| 16 | 10 | 40 | 394.1 | 3.1 | 0.368 | 0.042 |
| 17 | 10 | 60 | 375.2 | 1.4 | 0.350 | 0.043 |
| 18 | 2 | 60 | 484.5 | 3.5 | 0.429 | 0.009 |
| 19 | 6 | 40 | 667.5 | 6.8 | 0.410 | 0.013 |
| 20 | 2 | 40 | 482.3 | 7.9 | 0.429 | 0.004 |
| Run | Microfluidizer Processor | |||||
| Independent Variables | Response Variables | |||||
| Pressure (X1, MPa) | Cycles (X2) | Droplet Size (Y, nm) | Polydispersity Index (PDI) | |||
| Mean | SD | Mean | SD | |||
| 21 | 50 | 1 | 393.5 | 7.6 | 0.387 | 0.037 |
| 22 | 150 | 11 | 120.8 | 1.9 | 0.316 | 0.009 |
| 23 | 150 | 1 | 128.8 | 1.9 | 0.378 | 0.007 |
| 24 | 100 | 6 | 105.8 | 1.2 | 0.468 | 0.009 |
| 25 | 50 | 6 | 195.3 | 6.9 | 0.281 | 0.017 |
| 26 | 100 | 1 | 253.4 | 8.2 | 0.422 | 0.005 |
| 27 | 100 | 11 | 118.5 | 1.3 | 0.454 | 0.004 |
| 28 | 50 | 11 | 234.1 | 6.3 | 0.424 | 0.032 |
| 29 | 100 | 6 | 187.3 | 2.8 | 0.300 | 0.003 |
| 30 | 150 | 6 | 134.8 | 4.8 | 0.387 | 0.053 |
| Source | Regression Coefficients | F-Ratio | p-Value |
|---|---|---|---|
| a0 | 1029.33 | - | - |
| a1 | −46.5462 | 1.27 | 0.2841 |
| a2 | 75.8451 | 0.17 | 0.6882 |
| a3 | −47.5277 | 19.37 | 0.0011 |
| a4 | −171.888 | 9.00 | 0.0121 |
| a11 | −0.407612 | 0.02 | 0.8878 |
| a12 | 0.149121 | 0.02 | 0.8973 |
| a13 | 0.861198 | 2.05 | 0.1805 |
| a14 | 6.08568 | 4.08 | 0.0683 |
| a22 | −1.77558 | 0.40 | 0.5421 |
| a23 | −0.179323 | 0.09 | 0.7714 |
| a24 | −4.8638 | 2.61 | 0.1345 |
| a33 | 0.656502 | 0.67 | 0.4309 |
| a34 | 3.78764 | 5.56 | 0.0379 |
| a44 | 9.33476 | 0.22 | 0.6509 |
| Source | Rotor-Stator Mixer | Ultrasonic Homogenizer | Microfluidizer Processor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression Coefficients | F-Ratio | p-Value | Regression Coefficients | F-Ratio | p-Value | Regression Coefficients | F-Ratio | p-Value | |
| a0 | 2570.49 | - | - | 497.885 | - | - | 616.672 | - | - |
| a1 | −0.139724 | 2.06 | 0.2242 | −20.3173 | 3.44 | 0.1372 | −4.52892 | 13.05 | 0.0225 |
| a2 | −166.557 | 12.69 | 0.0235 | 7.39658 | 0.04 | 0.8426 | −48.2018 | 6.20 | 0.0675 |
| a11 | 2.7045 × 10−6 | 3.90 | 0.1195 | −1.80134 | 0.17 | 0.6993 | 0.0107943 | 0.69 | 0.4523 |
| a12 | 0.00341667 | 2.11 | 0.2203 | 0.546562 | 0.68 | 0.4558 | 0.1514 | 2.33 | 0.2014 |
| a22 | 3.05089 | 0.19 | 0.6827 | −0.139179 | 0.64 | 0.4675 | 1.91543 | 2.18 | 0.2140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niknam, S.M.; Escudero, I.; Benito, J.M. Formulation and Preparation of Water-In-Oil-In-Water Emulsions Loaded with a Phenolic-Rich Inner Aqueous Phase by Application of High Energy Emulsification Methods. Foods 2020, 9, 1411. https://doi.org/10.3390/foods9101411
Niknam SM, Escudero I, Benito JM. Formulation and Preparation of Water-In-Oil-In-Water Emulsions Loaded with a Phenolic-Rich Inner Aqueous Phase by Application of High Energy Emulsification Methods. Foods. 2020; 9(10):1411. https://doi.org/10.3390/foods9101411
Chicago/Turabian StyleNiknam, Seyed Mehdi, Isabel Escudero, and José M. Benito. 2020. "Formulation and Preparation of Water-In-Oil-In-Water Emulsions Loaded with a Phenolic-Rich Inner Aqueous Phase by Application of High Energy Emulsification Methods" Foods 9, no. 10: 1411. https://doi.org/10.3390/foods9101411