Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom
Abstract
:1. Introduction
2. Materials and Methods
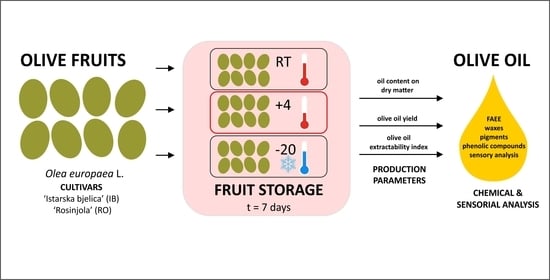
2.1. Olive Fruits, Storage Treatments and Virgin Olive Oil Production
2.2. Oil Content, Oil Yield and Extractability Index
2.3. Analysis of VOOs Pigments
2.4. Sensory Analysis
2.5. FAEE and Waxes
2.6. Analysis of Phenolic Compounds
2.7. Data Elaboration
3. Results and Discussion
3.1. Oil Content, Extractability Index and Ripening Index
3.2. VOO Pigments
3.3. Sensory Quality
3.4. FAEE and Waxes
3.5. Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.C. Fatty acid alkyl esters presence in olive oil vs. organoleptic assessment. Food Chem. 2012, 135, 1205–1209. [Google Scholar] [CrossRef]
- Vossen, P. Olive oil: History, production and characterization of the world’s classic oils. Hortscience 2007, 42, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Vichi, S.; Romero, A.; Gallardo-Chacón, J.; Tous, J.; López-Tamames, E.; Buxaderas, S. Influence of olives’ storage conditions on the formation of volatile phenols and their role in off-odor formation in the oil. J. Agric. Food Chem. 2009, 57, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Perez-Camino, M.C.; Moreda, W.; Mateos, R.; Cert, A. Determination of esters of fatty acids with low molecular weight alcohols in olive oils. J. Agric. Food Chem. 2002, 50, 4721–4725. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, M.; Bongratz, A.; Mariani, C.; Grob, K. Fatty acid methyl and ethyl esters as well as wax esters for evaluating the quality of olive oils. Eur. Food Res. Technol. 2008, 228, 65–74. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Regulation, H. European Economic Community. Commission Regulation (EEC) No 2568/91 of 11 July 1991 (and later modifications) on the characteristics of olive oil and olive-residue oil and the relevant methods of analysis. Off. J. Eur. Communities 1991, L248, 1–83. [Google Scholar]
- Hbaieb, R.H.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2014, 174, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Yousfi, K.; Weiland, C.M.; Garcia, J.M. Responses of fruit physiology and virgin oil quality to cold storage of mechanically harvested ‘Arbequina’ olives cultivated in hedgerow. Grasas Aceites 2013, 64, 572–582. [Google Scholar] [CrossRef] [Green Version]
- International Olive Council. Determination of the Content of Waxes, Fatty Acid Methyl Esters and Fatty Acid Ethyl Esters by Capillary Gas Chromatograhy; COI/T.20/Doc. No 28/Rev.2; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Boskou, D. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Press: Champaign, IL, USA, 2006. [Google Scholar]
- Servilli, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future–An overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhu, H.J.; Shoemaker, C.F.; Wang, S.C. The effect of different cold storage conditions on the compositions of extra virgin olive oil. J. Am. Oil Chem. Soc. 2014, 91, 1559–1570. [Google Scholar] [CrossRef]
- Hbaieb, R.H.; Kotti, F.; Cortes-Francisco, N.; Caixach, J.; Gargouri, M.; Vichi, S. Ripening and storage conditions of Chétoui and Arbequina olives: Part II. Effect on olive endogenous enzymes and virgin olive oil secoiridoid profile determined by high resolution mass spectrometry. Food Chem. 2016, 210, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Boim, S.; Sobotin, Y.; Zipori, I. Effect of mechanically harvested olive storage temperature and duration on oil quality. Horttechnology 2012, 22, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Lukić, I.; Horvat, I.; Godena, S.; Krapac, M.; Lukić, M.; Vrhovsek, U.; Brkić Bubola, K. Towards understanding the varietal typicity of virgin olive oil by correlating sensory and compositional analysis data: A case study. Food Res. Int. 2018, 112, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Belobrajić, I. Influence of storage temperature on quality parameters, phenols and volatile compounds of Croatian virgin olive oils. Grasas Aceites 2014, 65, e034. [Google Scholar] [CrossRef]
- García, J.M.; Yousfi, K. The postharvest of mill olives. Grassas Aceites 2006, 57, 16–24. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Delcuratolo, D.; Gomes, T.; Colelli, G. Effect of different temperatures and storage atmospheres on Coratina olive oil quality. Food Chem. 2007, 102, 571–576. [Google Scholar] [CrossRef]
- Garcia-Vico, L.; Garcia-Rodriguez, R.; Sanz, C.; Perez, A.G. Biochemical aspects of olive freezing-damage: Impact on the phenolic and volatile profiles of virgin olive oil. LWT-Food Sci. Technol. 2017, 86, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Yousfi, K.; Weiland, C.M.; Garcia, J.M. Effect of harvesting system and fruit cold storage on virgin olive oil chemical composition and quality of superintensive cultivated ‘arbequina’ olives. J. Agric. Food Chem. 2012, 60, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Changes in virgin olive oil quality during low-temperature fruit storage. J. Agric. Food Chem. 2008, 56, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Zhu, Z.W.; Sun, D.W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Masella, P.; Guerrini, L.; Angeloni, G.; Spadi, A.; Baldi, F.; Parenti, A. Freezing/storing olives, consequences for extra virgin olive oil quality. Int. J. Refrig. 2019, 106, 24–32. [Google Scholar] [CrossRef]
- Romero, I.; Aparicio-Ruiz, R.; Oliver-Pozo, C.; Aparicio, R.; Garcia-Gonzalez, D.L. Characterization of virgin olive oils with two kinds of ‘frostbitten olives’ sensory defect. J. Agric. Food Chem. 2016, 64, 5590–5597. [Google Scholar] [CrossRef]
- Hbaieb, R.H.; Kotti, F.; Gargouri, M.; Msallem, M.; Vichi, S. Ripening and storage conditions of Chétoui and Arbequina olives: Part. I. Effect on olive oils volatiles profile. Food Chem. 2016, 203, 548–558. [Google Scholar] [CrossRef]
- Poerio, A.; Bendini, A.; Cerretani, L.; Bonoli-Carbognin, M.; Lercker, G. Effect of olive fruit freezing on oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2008, 110, 368–372. [Google Scholar] [CrossRef]
- Beltrán, G.; Uceda, M.; Jiménez, A.; Aguilera, M.P. Olive oil extractability index as a parameter for olive cultivar characterisation. J. Sci. Food. Agric. 2003, 83, 503–506. [Google Scholar] [CrossRef]
- Brkić, K.; Radulović, M.; Sladonja, B.; Lukić, I.; Šetić, E. Application of soxtec apparatus for oil content determination in olive fruit. Riv. Ital. Sostanze Grasse 2006, 83, 115–119. [Google Scholar]
- Koprivnjak, O.; Brkić Bubola, K.; Kosić, U. Sodium chloride compared to talc as processing aid has similar impact on volatile compounds but more favorable on ortho-diphenols in virgin olive oil. Eur. J. Lipid Sci. Technol. 2016, 118, 318–324. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sánchez-Gòmez, A.H.; Garrido-Fernandez, J. Colour-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- International Olive Council. Sensory Analysis of Olive Oil: Method for the Organoleptic Assessment of Virgin Olive Oil; COI/T.20/Doc. No 15/Rev.10; International Olive Council: Madrid, Spain, 2018. [Google Scholar]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-Garcia, A.M.; Gomez-Rico, A.; Salvador, M.D.; Fregapane, G. Effect of preprocessing olive storage conditions on virgin olive oil quality and composition. J. Agric. Food Chem. 2010, 58, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Gutierrez, F.; Barrera, M.J.; Albi, M.A. Storage of mill olives on an industrial scale. J. Agric. Food Chem. 1996, 44, 590–593. [Google Scholar] [CrossRef]
- Garcia, J.M.; Gutiérrez, F.; Castellano, J.M.; Perdiguero, S.; Morilla, A.; Albi, M.A. Influence of storage temperature on fruit ripening and olive oil quality. J. Agric. Food Chem. 1996, 44, 264–267. [Google Scholar] [CrossRef]
- Morello, J.R.; Motilva, M.J.; Ramo, T.; Romero, M.P. Effect of freeze injuries in olive fruit on virgin olive oil composition. Food Chem. 2003, 81, 547–553. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Nanos, G.D.; Polymenopoulos, Z.; Thomai, T.; Sfakiotakis, E.M. Effect of fruit storage conditions on olive oil quality. J. Am. Oil Chem. Soc. 1998, 75, 721–724. [Google Scholar] [CrossRef]
- Guillaume, C.; Ravetti, L.; Gwyn, S. Characterisation of phenolic compounds in oils produced from frosted olives. J. Am. Oil Chem. Soc. 2010, 87, 247–254. [Google Scholar] [CrossRef]
- Romero, I.; Garcia-Gonzalez, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Study of volatile compounds of virgin olive oils with ‘frostbitten olives’ sensory defect. J. Agric. Food Chem. 2017, 65, 4314–4320. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Abdelhedi, R.; Bouaziz, M. Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 2015, 169, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The influence of the malaxation temperature on the activity of polyphenoloxidase and peroxidase and on the phenolic composition of virgin olive oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef] [PubMed]


| RI | % Oil on Dry Weight | Yield (%) | EI | |
|---|---|---|---|---|
| IB-control | 1.02 ± 0.10 b | 40.30 ± 1.60 | 10.03 ± 0.20 b | 0.45 ± 0.02 b |
| IB-RT | 1.73 ± 0.07 a | 37.95 ± 3.77 | 11.37 ± 0.38 a | 0.55 ± 0.04 a |
| IB+4 | 1.11 ± 0.05 b | 40.84 ± 1.02 | 9.88 ± 0.17 b | 0.44 ± 0.00 b |
| IB-20 | 1.16 ± 0.10 b | 40.90 ± 2.57 | 9.19 ± 0.42 b | 0.41 ± 0.03 b |
| RO-control | 1.64 ± 0.07 | 35.53 ± 2.76 | 5.11 ± 0.21 b | 0.22 ± 0.01 b |
| RO-RT | 1.75 ± 0.05 | 38.63 ± 4.51 | 7.14 ± 0.15 a | 0.29 ± 0.03 a |
| RO+4 | 1.72 ± 0.06 | 35.75 ± 1.95 | 5.25 ± 0.21 b | 0.23 ± 0.02 b |
| RO-20 | 1.58 ± 0.11 | 39.37 ± 3.00 | 5.33 ± 0.16 b | 0.21 ± 0.02 b |
| Ethyl Esters | Waxes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EE C16 | EE C18 | FAEE | C40 | C42 | C44 | C46 | C246 | C0246 | |
| IB-control | 2.58 ± 1.53 | 1.95 ± 0.87 c | 4.53 ± 1.8 b | 12.59 ± 0.76 c | 9.54 ± 0.98 c | 2.59 ± 0.22 b | 2.92 ± 0.72 | 15.06 ± 1.28 c | 27.64 ± 1.82 c |
| IB-RT | 3.48 ± 1.04 | 8.24 ± 0.82 a | 11.71 ± 0.28 a | 21.13 ± 1.66 a | 15.08 ± 0.95 a | 3.42 ± 0.15 a | 3.29 ± 0.25 | 21.79 ± 0.71 a | 42.92 ± 2.37 a |
| IB+4 | 2.71 ± 0.53 | 4.38 ± 1.14 b | 7.09 ± 1.61 b | 17.28 ± 0.42 b | 10.48 ± 0.27 bc | 2.67 ± 0.11 b | 2.37 ± 0.25 | 15.52 ± 0.39 c | 32.81 ± 0.80 b |
| IB-20 | 2.64 ± 0.12 | 3.56 ± 0.68 bc | 6.21 ± 0.56 b | 16.38 ± 1.26 b | 12.02 ± 0.80 b | 3.79 ± 0.49 a | 2.75 ± 0.31 | 18.56 ± 1.14 b | 34.94 ± 2.03 b |
| RO-control | 5.73 ± 1.10 | 2.44 ± 1.64 | 8.17 ± 2.74 | 14.59 ± 0.39 | 24.25 ± 6.67 ab | 5.79 ± 1.15 b | 1.46 ± 0.35 b | 31.49 ± 7.47 b | 46.08 ± 7.08 b |
| RO-RT | 3.18 ± 1.77 | 3.29 ± 1.68 | 6.47 ± 2.22 | 19.17 ± 2.44 | 34.21 ± 3.38 a | 12.59 ± 0.85 a | 3.85 ± 1.13 a | 50.65 ± 5.07 a | 69.82 ± 7.48 a |
| RO+4 | 2.88 ± 1.08 | 2.61 ± 1.88 | 5.49 ± 2.84 | 14.06 ± 2.47 | 22.84 ± 6.69 b | 8.42 ± 3.22 b | 1.80 ± 0.64 b | 33.06 ± 10.53 b | 47.12 ± 12.87 b |
| RO-20 | 2.96 ± 0.58 | 1.78 ± 0.93 | 4.73 ± 1.49 | 14.42 ± 2.47 | 20.44 ± 0.72 b | 6.62 ± 0.26 b | 1.79 ± 0.20 b | 28.85 ± 1.00 b | 43.28 ± 2.71 b |
| Phenolic Compounds (mg/kg) | IB-Control | IB-RT | IB+4 | IB-20 | RO-Control | RO-RT | RO+4 | RO-20 |
|---|---|---|---|---|---|---|---|---|
| Simple phenols | ||||||||
| hydroxytyrosol | 9.30 ± 0.96 b | 27.50 ± 6.48 a | 20.15 ± 0.90 a | 7.31 ± 2.83 b | 10.76 ± 1.51 b | 31.47 ± 2.62 a | 22.25 ± 5.72 a | 20.93 ± 5.40 ab |
| tyrosol | 7.67 ± 0.73 c | 40.16 ± 8.53 a | 20.22 ± 1.62 b | 11.42 ± 2.36 bc | 2.13 ± 0.26 b | 9.12 ± 3.53 a | 8.16 ± 2.07 ab | 3.62 ± 3.09 ab |
| hydroxytyrosol acetate | 1.14 ± 0.39 b | 2.86 ± 0.60 a | 2.01 ± 0.18 ab | 1.11 ± 0.27 b | 0.87 ± 0.24 | 1.31 ± 0.22 | 1.20 ± 0.09 | 0.85 ± 0.14 |
| vanillin | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.21 ± 0.01 | 0.20 ± 0.03 | 0.31 ± 0.04 | 0.25 ± 0.07 | 0.17 ± 0.06 | 0.20 ± 0.09 |
| Total simple phenols | 18.30 ± 0.88 c | 70.72 ± 15.19 a | 42.58 ± 0.53 b | 20.05 ± 5.36 c | 14.08 ± 2.03 b | 42.15 ± 5.87 a | 31.78 ± 7.82 a | 25.60 ± 8.27 ab |
| Secoiridoids | ||||||||
| secologanoside | 0.34 ± 0.09 | 0.21 ± 0.21 | 0.15 ± 0.18 | 0.24 ± 0.13 | 0.13 ± 0.14 | 0.18 ± 0.19 | 0.17 ± 0.08 | 0.26 ± 0.24 |
| elenolic acid glucoside (isomer) | 1.99 ± 0.46 a | 0.74 ± 1.13 ab | 0.44 ± 0.62 ab | 0.16 ± 0.10 b | 0.43 ± 0.67 | 0.73 ± 1.17 | 1.28 ± 1.07 | 0.47 ± 0.70 |
| 3,4-DHPEA-EDA | 120.02 ± 36.92 a | 29.58 ± 9.90 c | 84.78 ± 3.87 ab | 50.36 ± 4.75 bc | 73.30 ± 5.57 a | 48.63 ± 10.97 a | 58.65 ± 14.35 a | 35.30 ± 5.75 b |
| oleuropein aglycone (isomer I) | 62.34 ± 17.93 | 46.23 ± 3.49 | 52.90 ± 1.78 | 44.50 ± 9.08 | 152.05 ± 6.27 a | 98.64 ± 16.17 bc | 111.01 ± 7.86 bc | 73.26 ± 11.66 c |
| p-HPEA-EDA | 49.03 ± 8.82 a | 31.47 ± 5.70 b | 47.18 ± 2.80 a | 35.86 ± 1.82 ab | 11.24 ± 0.66 a | 10.13 ± 1.65 ab | 9.85 ± 2.20 ab | 6.79 ± 0.54 b |
| oleuropein + ligstroside aglycones I and II | 51.52 ± 4.84 a | 28.75 ± 5.73 b | 35.64 ± 2.18 b | 33.31 ± 3.16 b | 14.55 ± 0.60 a | 10.27 ± 0.99 bc | 12.05 ± 1.81 ab | 8.02 ± 1.61 c |
| oleuropein aglycone (isomer II) | 91.19 ± 15.37 a | 60.43 ± 2.27 b | 83.55 ± 4.17 ab | 69.99 ± 8.19 ab | 68.50 ± 2.39 | 72.68 ± 8.09 | 59.62 ± 5.95 | 62.16 ± 0.99 |
| ligstroside aglycon (isomer III) | 1.61 ± 0.21 b | 2.72 ± 0.78 ab | 3.59 ± 0.98 a | 4.11 ± 0.05 a | 1.41 ± 0.14 a | 1.52 ± 0.04 a | 1.53 ± 0.37 a | 0.80 ± 0.09 b |
| oleuropein aglycone (isomer III) | 9.93 ± 2.77 | 11.37 ± 1.23 | 14.87 ± 2.44 | 15.87 ± 3.04 | 6.33 ± 0.36 | 7.82 ± 1.00 | 7.20 ± 1.59 | 5.32 ± 0.49 |
| Total secoiridoids | 387.98 ± 71.14 a | 211.49 ± 22.77 c | 323.09 ± 13.86 ab | 254.41 ± 21.81 bc | 327.94 ± 8.77 a | 250.60 ± 33.36 b | 261.35 ± 35.28 ab | 192.38 ± 19.30 b |
| Lignans | ||||||||
| pinoresinol | 12.46 ± 2.11 b | 17.35 ± 0.37 a | 17.94 ± 0.06 a | 10.96 ± 0.40 b | 3.11 ± 0.11 a | 2.96 ± 0.04 a | 3.03 ± 0.02 a | 2.72 ± 0.08 b |
| acetoxypinoresinol | 24.85 ± 1.60 a | 24.84 ± 0.51 a | 25.80 ± 1.01 a | 20.99 ± 1.08 b | 30.25 ± 0.55 a | 30.66 ± 0.78 a | 30.59 ± 0.95 a | 25.53 ± 1.64 b |
| Total lignans | 37.31 ± 3.56 b | 42.20 ± 0.89 ab | 43.75 ± 1.08 a | 31.96 ± 0.72 c | 33.36 ± 0.66 a | 33.62 ± 0.75 a | 33.62 ± 0.93 a | 28.25 ± 1.60 b |
| Phenolic acids | ||||||||
| vanillic acid | 0.96 ± 0.09 b | 0.86 ± 0.08 b | 0.94 ± 0.01 b | 1.20 ± 0.04 a | 1.03 ± 0.03 | 1.05 ± 0.06 | 1.06 ± 0.10 | 1.03 ± 0.73 |
| p-coumaric acid | 1.74 ± 0.21 b | 5.16 ± 1.17 a | 2.12 ± 0.11 b | 1.28 ± 0.05 b | 0.46 ± 0.01 c | 4.74 ± 0.26 a | 1.26 ± 0.06 b | 0.43 ± 0.10 c |
| Total phenolic acids | 2.70 ± 0.27 b | 6.02 ± 1.11 a | 3.06 ± 0.10 b | 2.48 ± 0.05 b | 1.49 ± 0.04 b | 5.79 ± 0.32 a | 2.33 ± 0.16 b | 1.46 ± 0.71 b |
| Flavonoids | ||||||||
| luteolin | 1.96 ± 0.25 a | 1.14 ± 0.05 b | 1.43 ± 0.10 b | 0.70 ± 0.05 c | 0.96 ± 0.11 a | 0.77 ± 0.04 a | 0.90 ± 0.08 a | 0.49 ± 0.09 b |
| apigenin | 1.01 ± 0.09 a | 0.60 ± 0.01 bc | 0.72 ± 0.05 b | 0.48 ± 0.02 c | 0.35 ± 0.04 a | 0.29 ± 0.02 b | 0.34 ± 0.01 a | 0.24 ± 0.04 b |
| Total flavonoids | 2.97 ± 0.33 a | 1.74 ± 0.07 b | 2.15 ± 0.16 b | 1.18 ± 0.05 c | 1.32 ± 0.15 a | 1.06 ± 0.05 a | 1.24 ± 0.09 a | 0.73 ± 0.11 b |
| TOTAL PHENOLS | 449.26 ± 74.39 a | 332.17 ± 9.24 b | 414.63 ± 14.35 ab | 310.06 ± 24.78 b | 378.19 ± 9.16 a | 333.22 ± 28.63 a | 330.32 ± 28.64 a | 248.42 ± 18.35 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brkić Bubola, K.; Lukić, M.; Novoselić, A.; Krapac, M.; Lukić, I. Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom. Foods 2020, 9, 1445. https://doi.org/10.3390/foods9101445
Brkić Bubola K, Lukić M, Novoselić A, Krapac M, Lukić I. Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom. Foods. 2020; 9(10):1445. https://doi.org/10.3390/foods9101445
Chicago/Turabian StyleBrkić Bubola, Karolina, Marina Lukić, Anja Novoselić, Marin Krapac, and Igor Lukić. 2020. "Olive Fruit Refrigeration during Prolonged Storage Preserves the Quality of Virgin Olive Oil Extracted Therefrom" Foods 9, no. 10: 1445. https://doi.org/10.3390/foods9101445