Development of a Statistical Workflow for Screening Protein Extracts Based on Their Nutritional Composition and Digestibility: Application to Elderly
Abstract
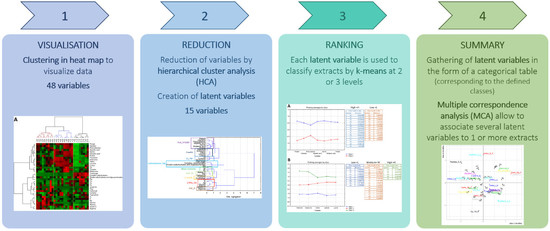
:1. Introduction
2. Materials and Methods
2.1. Protein Ingredient
2.2. Proximate Analysis
2.3. In Vitro Digestion of Protein Extracts
2.4. Bioactivity
2.5. Statistical Analysis
3. Results
3.1. Bioaccessibility and Bioactivity
3.2. Global Visualization of Variables and Protein Extracts
3.3. Reduction: Association of Quantitative Variables by Clustering
3.4. Classification of Protein Extracts to Obtain Categorical Value to Each Latent Variable
3.5. Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stratégie de Prise en Charge en cas de Dénutrition Protéino-Energétique chez la Personne Agée. Recommandation de Bonne Pratique. Available online: https://www.has-sante.fr/jcms/r_1495743/fr/strategie-de-prise-en-charge-en-cas-de-denutrition-proteino-energetique-chez-la-personne-agee (accessed on 30 June 2020).
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balage, M.; Averous, J.; Rémond, D.; Bos, C.; Pujos-Guillot, E.; Papet, I.; Mosoni, L.; Combaret, L.; Dardevet, D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J. Nutr. Biochem. 2010, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mosoni, L.; Balage, M.; Vazeille, E.; Combaret, L.; Morand, C.; Zagol-Ikapitte, I.; Boutaud, O.; Marzani, B.; Papet, I.; Dardevet, D. Antioxidant supplementation had positive effects in old rat muscle, but through better oxidative status in other organs. Nutrition 2010, 26, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M.; Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral Biol. 2005, 50, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Salles, N. Basic Mechanisms of the Aging Gastrointestinal Tract. Dig. Dis. 2007, 25, 112–117. [Google Scholar] [CrossRef]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef]
- Dangin, M.; Guillet, C.; Garcia-Rodenas, C.; Gachon, P.; Bouteloup-Demange, C.; Reiffers-Magnani, K.; Fauquant, J.; Ballèvre, O.; Beaufrère, B. The Rate of Protein Digestion affects Protein Gain Differently during Aging in Humans. J. Physiol. 2003, 549, 635–644. [Google Scholar] [CrossRef]
- Serafini, E.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Bernabei, R.; Landi, F. Nutritional approach to sarcopenia. J. Gerontol. Geriatr. 2019, 67, 52–61. [Google Scholar]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Harkouss, R.; Mirade, P.-S.; Gatellier, P. Development of a rapid, specific and efficient procedure for the determination of proteolytic activity in dry-cured ham: Definition of a new proteolysis index. Meat Sci. 2012, 92, 84–88. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, Y.; Buffière, C.; Cohade, B.; Vauris, M.; Liebermann, K.; Hafnaoui, N.; Lopez, M.; Souchon, I.; Dupont, D.; Rémond, D. True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods. Food Chem. 2021, 338, 128020. [Google Scholar] [CrossRef]
- Raes, K.; Knockaert, D.; Struijs, K.; Van Camp, J. Role of processing on bioaccessibility of minerals: Influence of localization of minerals and anti-nutritional factors in the plant. Trends Food Sci. Technol. 2014, 37, 32–41. [Google Scholar] [CrossRef]
- Rehman, Z.; Shah, W.H. Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Programme National Nutrition Santé 2019–2023. Available online: https://solidarites-sante.gouv.fr/IMG/pdf/pnns4_2019-2023.pdf (accessed on 30 June 2020).
- Landi, N.; Pacifico, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Mezzacapo, M.C.; Ragucci, S.; Iannuzzi, F.; Zarrelli, A.; Di Maro, A. Valle Agricola lentil, an unknown lentil (Lens culinaris Medik.) seed from Southern Italy as a novel antioxidant and prebiotic source. Food Funct. 2015, 6, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Keskin Ulug, S.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Siow, H.-L.; Lim, T.S.; Gan, C.-Y. Development of a workflow for screening and identification of α-amylase inhibitory peptides from food source using an integrated Bioinformatics-phage display approach: Case study—Cumin seed. Food Chem. 2017, 214, 67–76. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Sui, Z.; Niu, H.; Chen, L.; Hu, C.; Xuan, Q.; Hou, X.; Zhang, R.; Zhou, L.; et al. A multi-omics investigation of the molecular characteristics and classification of six metabolic syndrome relevant diseases. Theranostics 2020, 10, 2029–2046. [Google Scholar] [CrossRef]
- Buckee, G.K. Determination of total nitrogen in barley, malt and beer by Kjeldahl procedures and the dumas combustion methodcollaborative trial. J. Inst. Brew. 1994, 100, 57–64. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 47–53. ISBN 978-1-4419-1462-0. [Google Scholar]
- Poitevin, E. Official Methods for the Determination of Minerals and Trace Elements in Infant Formula and Milk Products: A Review. J. AOAC Int. 2016, 99, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, X. Fluorimetric determination of ascorbic acid with o-phenylenediamine. Talanta 2003, 59, 95–99. [Google Scholar] [CrossRef]
- Bayfield, R.F. Colorimetric determination of vitamin A with trichloroacetic acid. Anal. Biochem. 1971, 39, 282–287. [Google Scholar] [CrossRef]
- Biswas, A.K.; Sahoo, J.; Chatli, M.K. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar]
- Lynch, S.M.; Frei, B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J. Lipid Res. 1993, 34, 1745–1753. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Denis, S.; Sayd, T.; Georges, A.; Chambon, C.; Chalancon, S.; Santé-Lhoutellier, V.; Blanquet-Diot, S. Digestion of cooked meat proteins is slightly affected by age as assessed using the dynamic gastrointestinal TIM model and mass spectrometry. Food Funct. 2016, 7, 2682–2691. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Strug, I.; Utzat, C.; Cappione, A.; Gutierrez, S.; Amara, R.; Lento, J.; Capito, F.; Skudas, R.; Chernokalskaya, E.; Nadler, T. Development of a Univariate Membrane-Based Mid-Infrared Method for Protein Quantitation and Total Lipid Content Analysis of Biological Samples. J. Anal. Methods Chem. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Caraux, G.; Pinloche, S. PermutMatrix: A graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The Role of Milk- and Soy-Based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef]
- Hodgkinson, S.M.; Montoya, C.A.; Scholten, P.T.; Rutherfurd, S.M.; Moughan, P.J. Cooking Conditions Affect the True Ileal Digestible Amino Acid Content and Digestible Indispensable Amino Acid Score (DIAAS) of Bovine Meat as Determined in Pigs. J. Nutr. 2018, 148, 1564–1569. [Google Scholar] [CrossRef]
- Bax, M.-L.; Aubry, L.; Ferreira, C.; Daudin, J.-D.; Gatellier, P.; Rémond, D.; Santé-Lhoutellier, V. Cooking Temperature Is a Key Determinant of in Vitro Meat Protein Digestion Rate: Investigation of Underlying Mechanisms. J. Agric. Food Chem. 2012, 60, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Nunes, X.; Souza, F.; da S. Almeida, J.R.G.; de Lima, J.T.; de Arajo Ribeiro, L.A.; Quintans Jnior, L.J.; Barbosa Filho, J.M. Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0203-8. [Google Scholar]
- Karamać, M.; Kosińska, A.; Estrella, I.; Hernández, T.; Dueñas, M. Antioxidant activity of phenolic compounds identified in sunflower seeds. Eur. Food Res. Technol. 2012, 235, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Jallinoja, P.; Niva, M.; Latvala, T. Future of sustainable eating? Examining the potential for expanding bean eating in a meat-eating culture. Futures 2016, 83, 4–14. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Cao, K.-A.L.; Davis, M.J.; Déjean, S. Visualising associations between paired ‘omics’ data sets. BioData Min. 2012, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrAC Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Aceves, C.M.; Aksenov, A.A.; Aleti, G.; Almaliti, J.; Bouslimani, A.; Brown, E.A.; Campeau, A.; Caraballo-Rodríguez, A.M.; Chaar, R.; et al. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in raw and processed foods and beverages. Food Chem. 2020, 302, 125290. [Google Scholar] [CrossRef]




| Origin | Animal (A) | Dairy (D) | Plant (P) |
|---|---|---|---|
| 1 | Pork meat broth (hydrolyzed) ~ | Cheese powder ^ | Fava bean • |
| 2 | Pork heart ~ | 80% serum protein concentrate ^ | Fava bean (fermented) • |
| 3 | Pork broth ~ | 60% micellar casein concentrate ^ | Hemp * |
| 4 | Pork collagen ~ | Whey protein ^ | Pea * |
| 5 | Pork liver ~ | Whey protein ^ | Pea * |
| 6 | Pork liver ~ | Calcium caseinate * | Soybean * |
| 7 | Chicken broth ♦ | Casein rennet * | Soybean * |
| 8 | Chicken collagen ♦ | Milk protein concentrate * | Pumpkinseed * |
| 9 | Chicken meat ♦ | Whey protein * | Sunflower * |
| Sample | DM (%) | Protein (%) | Total Sugar (%) | Simple Carbohydrates and Oligosaccharides (%) | Complex Carbohydrates (%) | Cr (ppm) | Cu (ppm) | Zn (ppm) | Fe (ppm) | Na (ppm) | Mg (ppm) | K (ppm) | Ca (ppm) | Vitamin C (mg/g) | Vitamin A (µg/g) | TBARS (mg MDA/kg Powder) | Carbonyls (nM Hydrazone/mg Protein) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | A1 | 96.79 ± 0.86 | 83.46 ± 0.56 | 0.85 ± 0.01 | 1.12 ± 0.05 | n.r. | 0.28 ± 0.02 | 2.69 ± 0.14 | 26.58 ± 0.21 | 34.96 ± 7.55 | 12,142 ± 103 | 194 ± 10 | 1444 ± 32 | 549 ± 40 | 1.35 ± 0.07 | 151.93 ± 48.12 | 0.50 ± 0.02 | 17.24 ± 0.59 |
| A2 | 94.39 ± 0.44 | 66.10 ± 0.36 | 1.11 ± 0.03 | 0.74 ± 0.03 | 0.37 ± 0.04 | 0.12 ± 0.02 | 0.12 ± 0.02 | 1.84 ± 0.22 | 4.14 ± 0.40 | 5892 ± 132 | 428 ± 6 | 6584 ± 134 | 600 ± 17 | 0.99 ± 0.06 | n.r. | 12.40 ± 0.52 | 7.11 ± 0.13 | |
| A3 | 95.98 ± 0.25 | 85.38 ± 2.36 | 0.53 ± 0.03 | 1.05 ± 0.02 | n.r. | 0.13 ± 0.01 | 2.26 ± 0.03 | 34.02 ± 1.13 | 29.74 ± 0.19 | 1254 ± 16 | 203 ± 1 | 1065 ± 24 | 1544 ± 40 | 1.28 ± 0.09 | n.r. | 1.26 ± 0.13 | 14.99 ± 0.49 | |
| A4 | 97.10 ± 0.37 | 88.60 ± 1.06 | 0.51 ± 0.01 | 0.84 ± 0.02 | n.r. | 1.99 ± 0.34 | 0.74 ± 0.05 | 3.56 ± 0.03 | 17.60 ± 1.25 | 18,848 ± 3 | 592 ± 15 | 6796 ± 81 | 569 ± 19 | 1.25 ± 0.23 | n.r. | 0.33 ± 0.02 | 16.69 ± 0.41 | |
| A5 | 91.46 ± 0.50 | 66.50 ± 1.44 | 0.95 ± 0.03 | 1.17 ± 0.15 | n.r. | 0.49 ± 0.22 | 27.83 ± 1.67 | 202.21 ± 3.07 | 619.75 ± 8.81 | 8523 ± 373 | 541 ± 20 | 5955 ± 408 | 251 ± 13 | 0.17 ± 0.12 | 374.76 ± 2.56 | 22.21 ± 0.92 | 23.32 ± 1.64 | |
| A6 | 92.91 ± 0.17 | 65.85 ± 0.69 | 1.70 ± 0.05 | 0.97 ± 0.10 | 0.74 ± 0.03 | 0.01 ± 0.01 | 0.18 ± 0.03 | 0.06 ± 0.03 | 0.13 ± 0.04 | 6 ± 8 | 4 ± 2 | 9 ± 4 | 3 ± 0 | 0.58 ± 0.03 | 381.00 ± 59.98 | 3.36 ± 0.07 | 8.57 ± 0.10 | |
| A7 | 96.31 ± 0.10 | 61.13 ± 1.76 | 0.42 ± 0.03 | 1.56 ± 0.07 | n.r. | 0.40 ± 0.19 | 0.23 ± 0.07 | 39.19 ± 0.70 | 103.33 ± 44.88 | 2311 ± 100 | 535 ± 9 | 3193 ± 176 | 6521 ± 282 | 0.84 ± 0.07 | 88.89 ± 11.50 | 2.23 ± 0.13 | 15.57 ± 0.02 | |
| A8 | 95.83 ± 0.54 | 88.19 ± 0.44 | 0.86 ± 0.10 | 0.31 ± 0.09 | 0.54 ± 0.19 | 1.80 ± 0.06 | 6.72 ± 0.32 | 40.44 ± 1.11 | 201.35 ± 2.94 | 13,140 ± 156 | 762 ± 60 | 39,808 ± 46,613 | 331 ± 17 | 0.77 ± 0.15 | n.r. | 1.24 ± 0.07 | 10.63 ± 0.19 | |
| A9 | 94.16 ± 0.25 | 88.54 ± 1.00 | 0.48 ± 0.01 | 0.40 ± 0.04 | 0.09 ± 0.04 | 0.24 ± 0.03 | 0.01 ± 0.01 | 6.18 ± 0.89 | 9.20 ± 1.20 | 9539 ± 283 | 963 ± 23 | 8542 ± 1172 | 306 ± 2 | 0.67 ± 0.17 | n.r. | 0.36 ± 0.10 | 6.76 ± 0.36 | |
| Dairy | D1 | 97.82 ± 0.49 | 28.19 ± 0.63 | 9.28 ± 1.91 | 1.91 ± 0.16 | 7.37 ± 0.24 | 0.27 ± 0.01 | 0.50 ± 0.02 | 23.27 ± 0.09 | 9.68 ± 0.34 | 5850 ± 115 | 293 ± 1 | 3510 ± 14 | 7583 ± 5 | 0.36 ± 0.09 | n.r. | 0.22 ± 0.03 | 1.87 ± 0.17 |
| D2 | 92.79 ± 0.66 | 64.50 ± 0.63 | 1.61 ± 0.04 | 1.11 ± 0.18 | 0.50 ± 0.14 | 0.41 ± 0.01 | 2.14 ± 0.10 | 1.67 ± 0.04 | 6.89 ± 0.19 | 21,713 ± 556 | 68 ± 1 | 248 ± 9 | 698 ± 5 | 0.71 ± 0.10 | n.r. | n.r. | 8.38 ± 0.97 | |
| D3 | 95.62 ± 1.83 | 49.96 ± 2.02 | 20.82 ± 0.65 | 2.23 ± 0.13 | 18.60 ± 0.54 | 0.63 ± 0.01 | 0.74 ± 0.03 | 50.78 ± 0.46 | 5.51 ± 0.10 | 1485 ± 28 | 653 ± 9 | 6233 ± 68 | 16,313 ± NA | 0.20 ± 0.11 | n.r. | 0.01 ± 0.02 | 2.82 ± 0.19 | |
| D4 | 95.55 ± 0.11 | 76.19 ± 0.82 | 1.89 ± 0.32 | 0.29 ± 0.04 | 1.61 ± 0.34 | 0.08 ± 0.01 | 2.72 ± 0.16 | 2.32 ± 0.24 | 19.04 ± 1.08 | 2426 ± 60 | 518 ± 11 | 4825 ± 318 | 3197 ± 97 | 0.66 ± 0.08 | n.r. | 0.48 ± 0.06 | 9.21 ± 1.12 | |
| Dairy | D5 | 95.57 ± 0.63 | 85.42 ± 2.15 | 0.54 ± 0.05 | 0.32 ± 0.09 | 0.23 ± 0.14 | 0.11 ± 0.02 | 0.25 ± 0.04 | 0.39 ± 0.04 | 10.71 ± 2.43 | 542 ± 22 | 396 ± 10 | 527 ± 15 | 1564 ± 17 | 1.14 ± 0.08 | n.r. | 0.30 ± 0.10 | 11.47 ± 0.38 |
| D6 | 95.59 ± 0.49 | 91.13 ± 0.31 | 0.18 ± 0.02 | 0.30 ± 0.10 | n.r. | 0.06 ± 0.02 | 0.13 ± 0.03 | 20.82 ± 1.10 | 12.76 ± 3.18 | 49± 6 | 61 ± 11 | 44 ± 5 | 7587 ± 459 | 0.46 ± 0.04 | n.r. | n.r. | 4.53 ± 0.62 | |
| D7 | 88.81 ± 0.28 | 81.69 ± 0.57 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.03 | 0.14 ± 0.03 | 0.15 ± 0.03 | 76.52 ± 0.35 | 5.37 ± 1.22 | 52 ± 3 | 648 ± 9 | 122 ± 1 | 17,782 ± 666 | 1.05 ± 0.07 | n.r. | n.r. | 2.65 ± 0.16 | |
| D8 | 93.77 ± 0.44 | 81.63 ± 0.53 | 1.21 ± 0.15 | 1.47 ± 0.34 | n.r. | 0.27 ± 0.01 | 0.15 ± 0.01 | 66.29 ± 1.33 | 14.57 ± 1.39 | 6368 ± 99 | 484 ± 15 | 1336 ± 66 | 11,730 ± 66 | 0.48 ± 0.08 | n.r. | n.r. | 5.37 ± 0.71 | |
| D9 | 95.34 ± 1.55 | 77.75 ± 0.51 | 2.30 ± 0.21 | 1.01 ± 0.20 | 1.29 ± 0.04 | 0.12 ± 0.02 | 12.11 ± 1.19 | 77.71 ± 9.00 | 104.30 ± 7.98 | 3511 ± 332 | 5026 ± 51 | 4740 ± 1449 | 3471 ± 157 | 1.28 ± 0.02 | n.r. | 0.20 ± 0.10 | 4.71 ± 0.39 | |
| Plant | P1 | 39.05 ± 0.10 | 26.15 ± 0.19 | 9.46 ± 0.29 | 1.08 ± 0.08 | 8.39 ± 0.21 | 0.11 ± 0.01 | 8.13 ± 0.15 | 25.39 ± 0.05 | 26.38 ± 0.23 | 102 ± 42 | 661 ± 20 | 4362 ± 55 | 236 ± 4 | 1.02 ± 0.07 | 3.45 ± 0.07 | 1.47 ± 0.29 | 11.63 ± 0.76 |
| P2 | 43.07 ± 2.10 | 24.69 ± 1.88 | 7.05 ± 1.05 | 2.78 ± 0.10 | 4.28 ± 1.10 | 0.02 ± 0.01 | 6.29 ± 0.85 | 23.06 ± 1.46 | 22.64 ± 2.77 | 6 ± 2 | 579 ± 54 | 4448 ± 459 | 190 ± 8 | 1.48 ± 0.14 | 4.73 ± 0.10 | 0.57 ± 0.09 | 5.67 ± 1.03 | |
| P3 | 92.68 ± 0.64 | 49.13 ± NA | 12.31 ± 0.46 | 2.58 ± 0.14 | 9.74 ± 0.59 | 0.53 ± 0.05 | 16.45 ± 0.86 | 92.09 ± 4.16 | 173.13 ± 14.47 | 2 ± 0 | 5733 ± 198 | 5894 ± 46 | 1463 ± 81 | 0.69 ± 0.04 | 3.04 ± 0.39 | 7.33 ± 0.33 | 5.71 ± 0.13 | |
| P4 | 94.21 ± 0.36 | 84.79 ± 0.32 | 2.70 ± 0.06 | 1.32 ± 0.07 | 1.38 ± 0.13 | 0.17 ± 0.01 | 7.81 ± 0.01 | 49.29 ± 0.72 | 125.85 ± 5.31 | 6322 ± 0 | 471 ± 8 | 1744 ± 51 | 706 ± 23 | 0.85 ± 0.14 | 2.10 ± 0.17 | 2.14 ± 0.18 | 7.22 ± 0.30 | |
| P5 | 94.01 ± 0.87 | 69.89 ± 0.32 | 1.81 ± 0.02 | 1.39 ± 0.09 | 0.42 ± 0.07 | 0.38 ± 0.01 | 10.44 ± 0.11 | 61.54 ± 2.40 | 140.11 ± 1.78 | 4815 ± 128 | 405± 9 | 2183 ± 32 | 563 ± 6 | 1.71 ± 0.21 | 0.41 ± 0.01 | 1.14 ± 0.24 | 7.45 ± 0.76 | |
| P6 | 92.89 ± 1.18 | 58.61 ± 0.28 | 11.65 ± 0.57 | 0.52 ± 0.01 | 11.14 ± 0.56 | 0.61 ± 0.02 | 5.49 ± 0.14 | 24.12 ± 0.81 | 89.71 ± 0.20 | 68 ± 17 | 1665 ± 32 | 5423 ± 128 | 1598 ± 36 | 1.03 ± 0.03 | 0.17 ± 0.09 | 0.24 ± 0.04 | 4.96 ± 0.72 | |
| P7 | 96.24 ± 0.66 | 76.35 ± 0.97 | 1.71 ± 0.04 | 0.67 ± 0.24 | 1.05 ± 0.27 | 0.34 ± 0.02 | 10.10 ± 0.19 | 23.00 ± 0.90 | 97.34 ± 2.45 | 3150 ± 36 | 180 ± 8 | 4905 ± NA | 405 ± 6 | 1.24 ± 0.15 | 0.50 ± 0.04 | 1.65 ± 0.08 | 7.45 ± 1.13 | |
| P8 | 95.63 ± 0.67 | 57.81 ± 0.53 | 9.07 ± 0.12 | 1.02 ± 0.28 | 8.05 ± 0.26 | 0.15 ± 0.01 | 13.54 ± 0.83 | 87.02 ± 4.16 | 114.47 ± 6.40 | 3924 ± 252 | 5377 ± 446 | 5945 ± 256 | 690 ± 42 | 0.85 ± 0.14 | 6.19 ± 1.11 | 3.24 ± 0.06 | 10.51 ± 0.13 | |
| P9 | 91.07 ± 0.37 | 54.77 ± 0.10 | 12.80 ± 0.68 | 1.47 ± 0.22 | 11.33 ± 0.86 | 0.24 ± 0.04 | 28.86 ± 2.42 | 90.54 ± 7.07 | 85.49 ± 1.39 | 33 ± 45 | 4680 ± 310 | 5753 ± 812 | 1494 ± 201 | 1.37 ± 0.06 | 0.66 ± 0.38 | 0.36 ± 0.05 | 3.79 ± 0.05 |
| Sample | Indispensable Amino Acids—IAA (%DM) | Dispensable Amino Acids (%DM) | IAA/Total AA (%) | Total AA (%DM) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| His | Ile | Leu | Lys | Met | Phe | Thr | Trp | Val | Arg | Ala | Asp | Cys | Glu | Gly | Pro | Ser | Tyr | ||||
| Animal | A1 | 1.85 | 3.30 | 6.24 | 6.02 | 0.70 | 3.32 | 3.76 | 0.96 | 4.21 | 5.23 | 6.09 | 7.28 | 0.52 | 11.89 | 11.52 | 6.02 | 3.27 | 2.01 | 36.1 | 84.18 |
| A2 | 2.31 | 3.38 | 7.32 | 5.43 | 0.66 | 3.26 | 3.73 | 0.82 | 4.24 | 2.56 | 5.50 | 5.78 | 0.47 | 12.65 | 4.37 | 4.19 | 1.81 | 1.35 | 44.6 | 69.83 | |
| A3 | 1.99 | 3.23 | 6.48 | 6.08 | 0.31 | 3.50 | 3.80 | 0.87 | 4.60 | 5.12 | 6.52 | 7.29 | 0.40 | 11.06 | 11.02 | 7.42 | 3.23 | 2.05 | 36.3 | 84.98 | |
| A4 | 2.22 | 1.54 | 3.54 | 4.20 | 0.13 | 2.15 | 2.14 | 0.11 | 2.73 | 6.14 | 8.31 | 6.07 | 0.16 | 11.48 | 20.90 | 11.52 | 3.24 | 0.86 | 21.5 | 87.46 | |
| A5 | 1.63 | 3.77 | 7.19 | 5.88 | 0.62 | 3.85 | 3.62 | 1.81 | 4.73 | 0.89 | 4.52 | 6.57 | 0.57 | 9.86 | 4.72 | 4.31 | 3.02 | 0.99 | 48.3 | 68.55 | |
| A6 | 1.68 | 4.09 | 7.98 | 2.28 | 0.84 | 4.02 | 2.88 | 1.26 | 5.25 | 1.55 | 5.02 | 7.09 | 0.52 | 9.89 | 4.82 | 3.88 | 2.25 | 1.45 | 45.4 | 66.74 | |
| A7 | 1.83 | 3.12 | 5.77 | 5.52 | 0.20 | 2.90 | 3.37 | 0.84 | 3.31 | 3.65 | 4.51 | 6.15 | 0.15 | 9.90 | 6.28 | 3.99 | 2.65 | 2.11 | 40.5 | 66.26 | |
| A8 | 1.83 | 2.73 | 5.54 | 5.25 | 0.49 | 2.87 | 3.93 | 0.35 | 3.42 | 5.96 | 7.12 | 6.77 | 0.36 | 12.14 | 14.41 | 7.85 | 3.06 | 1.95 | 30.7 | 86.02 | |
| A9 | 3.20 | 3.68 | 6.77 | 6.89 | 0.61 | 3.64 | 4.27 | 0.56 | 3.88 | 4.08 | 7.22 | 7.96 | 0.97 | 14.25 | 11.03 | 5.25 | 3.19 | 2.49 | 37.2 | 89.95 | |
| Dairy | D1 | 0.95 | 1.60 | 2.90 | 2.21 | 0.42 | 1.58 | 1.24 | 0.62 | 1.80 | 0.92 | 0.97 | 2.06 | 0.85 | 6.20 | 0.56 | 3.42 | 1.47 | 1.50 | 42.6 | 31.26 |
| D2 | 1.12 | 6.18 | 5.72 | 5.99 | 0.22 | 1.55 | 7.25 | 0.66 | 5.68 | 2.72 | 5.72 | 9.89 | 0.51 | 20.36 | 1.08 | 10.98 | 5.52 | 1.09 | 37.3 | 92.24 | |
| D3 | 1.26 | 1.52 | 4.51 | 5.87 | 0.66 | 2.36 | 2.31 | 0.95 | 1.89 | 2.79 | 1.52 | 4.11 | 0.92 | 11.23 | 1.77 | 11.89 | 2.22 | 1.72 | 35.8 | 59.50 | |
| D4 | 1.27 | 4.89 | 9.04 | 8.82 | 0.61 | 2.33 | 5.62 | 1.51 | 4.62 | 1.68 | 4.30 | 5.13 | 0.97 | 14.08 | 1.45 | 4.23 | 3.35 | 1.75 | 51.2 | 75.65 | |
| D5 | 1.65 | 5.59 | 9.85 | 7.73 | 1.24 | 2.96 | 6.34 | 0.24 | 5.03 | 1.86 | 4.51 | 8.83 | 0.47 | 15.16 | 1.59 | 5.20 | 3.89 | 2.00 | 48.3 | 84.13 | |
| D6 | 2.76 | 4.40 | 9.05 | 7.15 | 1.34 | 4.85 | 3.86 | 0.20 | 5.63 | 2.51 | 2.74 | 6.30 | 0.37 | 20.10 | 1.61 | 8.99 | 4.95 | 3.85 | 43.3 | 90.65 | |
| D7 | 2.53 | 3.59 | 8.67 | 6.69 | 1.58 | 4.55 | 3.30 | 0.59 | 4.87 | 2.32 | 2.28 | 6.06 | 0.33 | 19.38 | 1.57 | 8.99 | 4.93 | 4.25 | 42.0 | 86.47 | |
| D8 | 2.40 | 4.26 | 8.53 | 6.67 | 1.61 | 4.08 | 3.91 | 1.17 | 5.19 | 1.51 | 2.71 | 6.24 | 0.67 | 17.94 | 1.46 | 7.96 | 4.38 | 3.36 | 45.0 | 84.05 | |
| D9 | 1.61 | 5.35 | 9.26 | 7.74 | 0.72 | 2.76 | 6.44 | 1.68 | 4.87 | 1.52 | 4.26 | 9.19 | 0.98 | 14.94 | 1.49 | 4.69 | 3.86 | 2.20 | 48.4 | 83.56 | |
| Plant | P1 | 0.98 | 1.38 | 2.37 | 1.89 | 0.48 | 1.38 | 1.09 | 0.11 | 1.35 | 2.46 | 1.23 | 2.46 | 0.62 | 4.66 | 1.30 | 1.59 | 1.62 | 1.02 | 39.4 | 27.98 |
| P2 | 0.89 | 1.55 | 3.05 | 2.05 | 0.38 | 1.51 | 1.11 | 0.12 | 1.65 | 1.15 | 1.28 | 2.66 | 0.52 | 4.89 | 1.25 | 1.59 | 1.56 | 1.18 | 43.3 | 28.38 | |
| P3 | 1.40 | 1.85 | 3.55 | 2.09 | 0.64 | 2.49 | 2.24 | 0.61 | 5.59 | 4.50 | 2.49 | 5.65 | 0.59 | 9.95 | 2.49 | 2.52 | 2.68 | 1.86 | 38.4 | 53.19 | |
| P4 | 1.98 | 5.71 | 9.83 | 7.86 | 0.24 | 2.89 | 6.14 | 0.77 | 5.32 | 1.98 | 4.52 | 8.89 | 0.55 | 14.89 | 1.52 | 5.18 | 3.68 | 1.89 | 48.6 | 83.84 | |
| P5 | 1.98 | 2.72 | 7.02 | 8.89 | 0.51 | 4.23 | 3.42 | 0.88 | 3.25 | 6.42 | 3.55 | 10.25 | 0.45 | 16.72 | 3.42 | 8.12 | 4.52 | 2.55 | 37.0 | 88.90 | |
| P6 | 1.78 | 2.19 | 5.42 | 7.42 | 0.61 | 3.52 | 3.12 | 0.72 | 2.31 | 4.89 | 3.15 | 9.15 | 0.42 | 15.62 | 2.78 | 8.13 | 3.55 | 2.25 | 35.2 | 77.03 | |
| P7 | 2.42 | 3.56 | 8.12 | 6.85 | 0.52 | 5.52 | 4.52 | 0.75 | 3.99 | 7.05 | 4.36 | 9.22 | 0.48 | 20.12 | 4.22 | 9.12 | 5.21 | 2.63 | 36.7 | 98.66 | |
| P8 | 1.37 | 2.32 | 4.76 | 2.30 | 0.49 | 3.52 | 2.36 | 0.91 | 2.89 | 6.08 | 2.98 | 6.01 | 0.42 | 12.50 | 3.61 | 2.50 | 3.19 | 2.14 | 34.7 | 60.35 | |
| P9 | 1.64 | 2.45 | 4.05 | 2.06 | 0.58 | 3.02 | 2.65 | 0.87 | 3.00 | 6.52 | 2.45 | 5.65 | 0.59 | 13.25 | 3.42 | 3.06 | 2.51 | 1.68 | 34.2 | 59.45 | |
| Sample | Protein (mg/g Powder) | Peptides (mg/g Powder) | Free NH2 (mM Glycine Equivalent/g Powder) | Inhibition of ABTS Radical (%) | Antioxidant Power to Reduce Ferric Ions (% Inhibition) | Inhibition of DPPH Radical (%) | ORAC (µmol Trolox Equivalent/mg Peptide Digestate) | |
|---|---|---|---|---|---|---|---|---|
| Animal | A1 | 546 ± 23 | 541 ± 54 | 4317 ± 620 | 26.358 ± 2.09 | 5.31 ± 0.41 | 51.57 ± 2.56 | 0.11 ± 0.07 |
| A2 | 478 ± 141 | 435 ± 26 | 1858 ± 203 | 37.99 ± 1.47 | 6.09 ± 0.66 | 47.59 ± 1.46 | 0.16 ± 0.04 | |
| A3 | 558 ± 83 | 215 ± 40 | 191 ± 16 | 25.23 ± 2.39 | 5.63 ± 0.47 | 16.72 ± 11.10 | 1.13 ± 0.33 | |
| A4 | 516 ± 26 | 568 ± 21 | 1794 ± 259 | 30.98 ± 0.55 | 6.27 ± 0.11 | 60.97 ± 0.56 | 0.34 ± 0.09 | |
| A5 | 364 ± 20 | 407 ± 37 | 2285 ± 914 | 77.63 ± 3.73 | 15 ± 8.69 | 72.41 ± 3.53 | 0.09 ± 0.02 | |
| A6 | 269 ± 30 | 459 ± 35 | 2152 ± 370 | 94.66 ± 2.18 | 39.83 ± 1.51 | 79.45 ± 1.73 | 0.06 ± 0.02 | |
| A7 | 1064 ± 233 | 276 ± 23 | 334 ± 49 | 24.79 ± 1.00 | 5.97 ± 0.24 | 9.15 ± 2.39 | 0.90 ± 0.28 | |
| A8 | 536 ± 33 | 663 ± 180 | 1942 ± 856 | 60.39 ± 1.97 | 16.66 ± 0.25 | 44.6 ± 3.11 | 0.06 ± 0.01 | |
| A9 | 370 ± 25 | 571 ± 41 | 3458 ± 604 | 73.82 ± 5.01 | 16.41 ± 0.71 | 62.08 ± 4.61 | 0.06 ± 0.02 | |
| Dairy | D1 | 800 ± 237 | 153 ± 7 | 208 ± 138 | 15.57 ± 0.61 | 4.24 ± 0.65 | 54.22 ± 3.61 | 0.29 ± 0.19 |
| D2 | 722 ± 17 | 231 ± 77 | 938 ± 84 | 15.8 ± 0.52 | 5.35 ± 0.18 | 21.72 ± 7.10 | 0.3 ± 0.07 | |
| D3 | 483 ± 11 | 211 ± 19 | 231 ± 10 | 18.36 ± 0.30 | 4.49 ± 0.16 | 57.87 ± 1.32 | 0.14 ± 0.03 | |
| D4 | 901 ± 52 | 246 ± 17 | 569 ± 64 | 15.1 ± 0.70 | 6.38 ± 0.45 | 52.89 ± 1.94 | 0.31 ± 0.23 | |
| D5 | 1018 ± 38 | 262 ± 22 | 393 ± 63 | 11.45 ± 0.36 | 0.99 ± 0.22 | 48.16 ± 2.82 | 0.6 ± 0.32 | |
| D6 | 839 ± 99 | 298 ± 24 | 318 ± 49 | 12.84 ± 0.54 | 2.11 ± 0.21 | 63.68 ± 9.12 | 0.27 ± 0.09 | |
| D7 | 462 ± 74 | 324 ± 32 | 506 ± 131 | 10.78 ± 0.44 | 1.69 ± 0.144 | 56.53 ± 2.92 | 0.24 ± 0.05 | |
| D8 | 698 ± 32 | 278 ± 34 | 367 ± 141 | 12.68 ± 0.76 | 1.95 ± 0.72 | 63.67 ± 3.75 | 1.02 ± 0.4 | |
| D9 | 869 ± 51 | 390 ± 43 | 967 ± 310 | 14.13 ± 0.44 | 3.60 ± 0.22 | 49.25 ± 3.66 | 0.38 ± 0.11 | |
| Plant | P1 | 219 ± 13 | 94 ± 6 | 438 ± 53 | 88.71 ± 0.33 | 42.90 ± 1.02 | 75.49 ± 1.92 | 0.02 ± 0.04 |
| P2 | 203 ± 18 | 382 ± 77 | 408 ± 17 | 88.84 ± 1.65 | 43.06 ± 4.33 | 71.11 ± 3.85 | 0.11 ± 0.01 | |
| P3 | 431 ± 12 | 246 ± 20 | 534 ± 102 | 47.63 ± 8.54 | 11.14 ± 2.91 | 22.01 ± 14.23 | 0.29 ± 0.10 | |
| P4 | 859 ± 33 | 334 ± 33 | 464 ± 31 | 20.95 ± 0.98 | 3.86 ± 0.22 | 44.86 ± 1.07 | 0.29 ± 0.07 | |
| P5 | 874 ± 59 | 187 ± 25 | 406 ± 15 | 14.61 ± 0.59 | 2.36 ± 0.03 | 41.79 ± 0.82 | 0.32 ± 0.07 | |
| P6 | 675 ± 92 | 140 ± 36 | 367 ± 62 | 21.86 ± 1.68 | 9.73 ± 0.35 | 9.61 ± 9.94 | 1.60 ± 0.78 | |
| P7 | 628 ± 183 | 170 ± 38 | 645 ± 62 | 21.69 ± 0.44 | 9.33 ± 0.48 | 31.04 ± 3.71 | 0.17 ± 0.21 | |
| P8 | 295 ± 29 | 261 ± 29 | 289 ± 42 | 39.63 ± 5.04 | 29.04 ± 5.78 | 32.18 ± 8.97 | 0.26 ± 0.09 | |
| P9 | 505 ± 26 | 235 ± 16 | 608 ± 62 | 82.09 ± 3.67 | 15.51 ± 3.86 | 38.15 ± 4.87 | 0.08 ± 0.04 |
| Sample | Protein (mg/g Powder) | Peptides (mg/g Powder) | Free NH2 (mM Glycine Equivalent/g Powder) | Inhibition of ABTS Radical (%) | Antioxidant Power to Reduce Ferric Ions (% Inhibition) | Inhibition of DPPH Radical (%) | ORAC (µmol Trolox Equivalent/mg Peptide Digestate) | |
|---|---|---|---|---|---|---|---|---|
| Animal | A1 | 496 ± 80 | 839 ± 151 | 6199 ± 1039 | 15.71 ± 1.17 | 3.99 ± 0.35 | 44.64 ± 1.64 | 0.15 ± 0.07 |
| A2 | 250 ± 22 | 670 ± 29 | 3418 ± 640 | 26.62 ± 0.92 | 5.11 ± 0.21 | 48.87 ± 0.49 | 0.46 ± 0.11 | |
| A3 | 551 ± 47 | 733 ± 63 | 2454 ± 398 | 30.16 ± 1.07 | 7.00 ± 0.29 | 51.30 ± 0.63 | 0.03 ± 0.01 | |
| A4 | 461 ± 32 | 859 ± 59 | 2642 ± 332 | 18.28 ± 1.23 | 4.66 ± 0.27 | 53.44 ± 2.83 | 1.26 ± 0.33 | |
| A5 | 271 ± 19 | 660 ± 54 | 3257 ± 382 | 51.42 ± 3.59 | 17.45 ± 1.07 | 65.20 ± 2.14 | 0.05 ± 0.04 | |
| A6 | 187 ± 35 | 739 ± 76 | 3791 ± 773 | 70.33 ± 3.82 | 26.11 ± 1.29 | 66.89 ± 2.02 | 0.10 ± 0.01 | |
| A7 | 549 ± 59 | 546 ± 48 | 1661 ± 397 | 21.02 ± 1.94 | 6.73 ± 0.37 | 56.00 ± 1.27 | 0.40 ± 0.11 | |
| A8 | 418 ± 31 | 1213 ± 324 | 2362 ± 265 | 35.20 ± 1.37 | 10.55 ± 0.36 | 33.00 ± 2.21 | 0.10 ± 0.06 | |
| A9 | 341 ± 87 | 870 ± 181 | 5142 ± 392 | 43.77 ± 1.08 | 10.47 ± 0.65 | 59.93 ± 1.71 | 0.17 ± 0.08 | |
| Dairy | D1 | 763 ± 91 | 333 ± 27 | 798 ± 366 | 5.76 ± 6.41 | 5.13 ± 0.83 | 25.65 ± 1.86 | 0.02 ± 0.02 |
| D2 | 568 ± 85 | 464 ± 27 | 2559 ± 844 | 16.17 ± 0.55 | 5.91 ± 0.44 | 33.16 ± 5.98 | 0.12 ± 0.04 | |
| D3 | 335 ± 8 | 530 ± 33 | 2188 ± 89 | 14.37 ± 0.29 | 4.59 ± 0.07 | 20.82 ± 1.54 | 0.06 ± 0.04 | |
| D4 | 525 ± 53 | 604 ± 62 | 2486 ± 1415 | 18.33 ± 0.65 | 13.80 ± 0.46 | 44.11 ± 1.01 | 0.11 ± 0.07 | |
| D5 | 563 ± 91 | 837 ± 74 | 3529 ± 651 | 14.38 ± 0.86 | 2.14 ± 0.11 | 34.96 ± 3.52 | 0.13 ± 0.05 | |
| D6 | 505 ± 29 | 854 ± 50 | 3433 ± 678 | 11.26 ± 0.53 | 2.25 ± 0.19 | 35.49 ± 4.46 | 0.07 ± 0.01 | |
| D7 | 412 ± 159 | 861 ± 63 | 5146 ± 430 | 10.89 ± 0.35 | 2.03 ± 0.09 | 24.96 ± 0.78 | 0.04 ± 0.04 | |
| D8 | 452 ± 24 | 786 ± 40 | 3004 ± 456 | 13.21 ± 0.31 | 2.87 ± 0.20 | 31.98 ± 1.45 | 0.19 ± 0.08 | |
| D9 | 507 ± 37 | 819 ± 106 | 4205 ± 655 | 16.58 ± 1.60 | 4.45 ± 0.16 | 44.03 ± 1.62 | 0.17 ± 0.02 | |
| Plant | P1 | 144 ± 21 | 253 ± 17 | 1000 ± 192 | 80.10 ± 0.66 | 38.49 ± 0.78 | 76.62 ± 1.87 | 0.09 ± 0.01 |
| P2 | 186 ± 189 | 771 ± 48 | 650 ± 91 | 67.21 ± 5.70 | 43.21 ± 1.93 | 71.85 ± 0.90 | 0.17 ± 0.04 | |
| P3 | 325 ± 22 | 586 ± 44 | 2511 ± 279 | 74.90 ± 6.27 | 22.60 ± 1.49 | 38.91 ± 7.70 | 0.13 ± 0.03 | |
| P4 | 541 ± 67 | 719 ± 95 | 2158 ± 94 | 25.30 ± 1.24 | 6.46 ± 0.16 | 45.33 ± 0.91 | 0.22 ± 0.04 | |
| P5 | 502 ± 36 | 554 ± 56 | 3264 ± 106 | 22.16 ± 0.36 | 6.04 ± 0.13 | 39.38 ± 1.62 | 0.14 ± 0.02 | |
| P6 | 418 ± 15 | 423 ± 40 | 3059 ± 156 | 28.80 ± 1.11 | 13.33 ± 0.44 | 21.81 ± 5.78 | 0.51 ± 0.52 | |
| P7 | 463 ± 109 | 506 ± 104 | 4968 ± 242 | 35.26 ± 0.96 | 13.85 ± 0.90 | 47.83 ± 2.61 | 0.36 ± 0.06 | |
| P8 | 310 ± 20 | 808 ± 194 | 2372 ± 354 | 39.08 ± 6.24 | 22.60 ± 1.49 | 39.84 ± 3.22 | 0.08 ± 0.12 | |
| P9 | 300 ± 18 | 628 ± 48 | 2403 ± 325 | 82.56 ± 4.56 | 82.78 ± 2.08 | 66.40 ± 4.14 | 0.06 ± 0.03 |
| HCA | k-Means | |||
|---|---|---|---|---|
| Variable | Latent Variable | High (H) | Low (L) | Medium (M) |
| Direct detect | Peptides_G | A1; A3; A5; A6; A7; A8; A9 | A2; A4; D1; D2; D3; D4; D5; D6; D7; D8; D9; P1; P2; P3; P4; P5; P6; P7; P8; P9 | |
| Fluorescamine | ||||
| %ABTS | Antiox_G | A5; A7; A8; A9; P2; P3; P4; P9 | A1; A2; A3; A4; A6; D1; D2; D3; D4; D5; D6; D7; D8; D9; P1; P5; P6; P7; P8 | |
| %FRAP | ||||
| %DPPH | ||||
| Biuret | BiuretORAC_G | A2; A4; D1; D2; D3; D5; D8; D9; P1; P7; P8 | A1; A3; A5; A6; D4; D6; D7; P5; P6; P9 | A7; A8; A9; P2; P3; P4 |
| ORAC | ||||
| Direct detect | Peptides_I | A1; A4; A6; A8; A9; D5; D6; D7; D8; D9 | A7; D1; P1; P2 | A2; A3; A5; D2; D3; D4; P3; P4; P5; P6; P7; P8; P9 |
| Fluorescamine | ||||
| %ABTS | Antiox_I | A6; P1; P2; P9 | A8; D1; D2; D3; D5; D6; D7; D8; P5; P6 | A1; A2; A3; A4; A5; A7; A9; D4; D9; P3; P4; P7; P8 |
| %FRAP | ||||
| %DPPH | ||||
| Biuret | BiuretORAC_I | A1; A3; A4; A7; D1; D2; D4; D5; D6; D8; D9; P4; P5; P6; P7 | A2; A5; A6; A8; A9; D3; D7; P1; P2; P3; P8; P9 | |
| ORAC | ||||
| %Protein | Prot_HFDSEP | A1; A3; A4; A8; A9; D2; D5; D6; D7; D8; D9; P4; P5; P6; P7 | A2; A5; A6; A7; D1; D3; D4; P1; P2; P3; P8; P9 | |
| Histidine | ||||
| Phenyalanine | ||||
| Aspartic acid | ||||
| Serine | ||||
| Glutamic acid | ||||
| Proline | ||||
| Threonine | TIVLK | D2; D5; D4; D9; P4 | A1; A2; A3; A5; A6; A7; A8; A9; D6; D7; D8; P5; P6; P7 | A4; D1; D3; P1; P2; P3; P8; P9 |
| Isoleucine | ||||
| Valine | ||||
| Leucine | ||||
| Lysine | ||||
| Ca | Ca_YM | D3; D6; D7; D8 | A1; A2; A3; A4; A5; A6; A7; A8; A9; D1; D2; D4; D5; D9; P1; P2; P3; P4; P5; P6; P7; P8; P9 | |
| Tyrosine | ||||
| Methionine | ||||
| Total sugar | Carbohydrates_Mg | D1; D3; P1; P2; P3; P6; P8; P9 | A1; A2; A3; A4; A5; A6; A7; A8; A9; D2; D4; D5; D6; D7; D8; D9; P4; P5; P7 | |
| Complex carbohydrates | ||||
| Simple carbohydrates and oligosaccharides | ||||
| Mg | ||||
| Cu | Micro-nutrients | A5; D1; D9; P3; P5; P8; P9 | A1; A2; A3; A4; A6; A7; A8; A9; D2; D3; D4; D5; D6; D7; D8; P1; P2; P4; P6; P7 | |
| Zn | ||||
| Fe | ||||
| Vitamin A | VitA_Ox | A5; A6 | A1; A2; A3; A4; A7; A8; A9; D1; D2; D3; D4; D5; D6; D7; D8; D9; P1; P2; P3; P4; P5; P6; P7; P8; P9 | |
| TBARS | ||||
| Carbonyles | ||||
| Cysteine | C and W | A9; D1; D3; D4; D9 | A1; A2; A3; A5; A6; A7; D2; D8; P3; P4; P5; P6; P7; P8; P9 | A4; A8; D5; D6; D7; P1; P2 |
| Tryptophan | ||||
| Cr | CrKNa_GA | A4; A8 | A1; A2; A3; A5; A6; A7; A9; D1; D2; D3; D4; D5; D6; D7; D8; D9; P1; P2; P3; P4; P5; P6; P7; P8; P9 | |
| K | ||||
| Na | ||||
| Glycine | ||||
| Alanine | ||||
| Vitamin C | VitC_R | A1; A2; A3; A4; A7; A8; D5; D7; D9; P1; P2; P3; P5; P6; P7; P8; P9 | A5; A6; A9; D1; D2; D3; D4; D6; D8; P4 | |
| Arginine | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duval, A.; Sayd, T.; Aubry, L.; Ferreira, C.D.O.; Ferraro, V.; Sante-Lhoutellier, V. Development of a Statistical Workflow for Screening Protein Extracts Based on Their Nutritional Composition and Digestibility: Application to Elderly. Foods 2020, 9, 1499. https://doi.org/10.3390/foods9101499
Duval A, Sayd T, Aubry L, Ferreira CDO, Ferraro V, Sante-Lhoutellier V. Development of a Statistical Workflow for Screening Protein Extracts Based on Their Nutritional Composition and Digestibility: Application to Elderly. Foods. 2020; 9(10):1499. https://doi.org/10.3390/foods9101499
Chicago/Turabian StyleDuval, Angéline, Thierry Sayd, Laurent Aubry, Claude De Oliviera Ferreira, Vincenza Ferraro, and Véronique Sante-Lhoutellier. 2020. "Development of a Statistical Workflow for Screening Protein Extracts Based on Their Nutritional Composition and Digestibility: Application to Elderly" Foods 9, no. 10: 1499. https://doi.org/10.3390/foods9101499
APA StyleDuval, A., Sayd, T., Aubry, L., Ferreira, C. D. O., Ferraro, V., & Sante-Lhoutellier, V. (2020). Development of a Statistical Workflow for Screening Protein Extracts Based on Their Nutritional Composition and Digestibility: Application to Elderly. Foods, 9(10), 1499. https://doi.org/10.3390/foods9101499