Gastrointestinal In Vitro Digests of Infant Biscuits Formulated with Bovine Milk Proteins Positively Affect In Vitro Differentiation of Human Osteoblast-Like Cells
Abstract
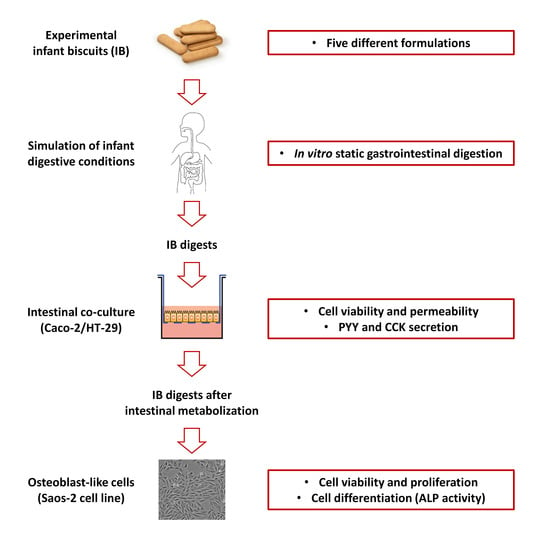
:1. Introduction
2. Materials and Methods
2.1. Formulation of Experimental IBs
2.2. In Vitro Gastrointestinal Digestion of IBs
2.3. Human In Vitro Intestinal and Osteoblast-Like Cells
2.4. Effects of IBDs on Intestinal Co-Culture Cell Viability and Monolayer Integrity
2.5. PYY and CCK Secretion Induced by IBDs
2.6. Effects of MIBDs on Osteoblast Viability and Cell Proliferation
2.7. Osteoblast ALP (EC 3.1.3.1) Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Effects of IBDs on Intestinal Cell Co-Culture
3.2. In Vitro Effects of MIBDs on Saos-2 Cell Culture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guimont, C.; Marchall, E.; Girardet, J.M.; Linden, G.; Otani, H. Biologically active factors in bovine milk and dairy byproducts: Influence on cell culture. Crit. Rev. Food Sci. Nutr. 1997, 37, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Budek, A.Z.; Hoppe, C.; Ingstrup, H.; Michaelsen, K.F.; Bügel, S.; Mølgaard, C. Dietary protein intake and bone mineral content in adolescents—The Copenhagen Cohort Study. Osteoporos. Int. 2007, 18, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: Dairy intake and bone health: A viewpoint from the state of the art1. J. Dairy Sci. 2011, 94, 5249–5262. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Lazarenko, O.P.; Blackburn, M.L.; Badeaux, J.V.; Badger, T.M.; Ronis, M.J.J. Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J. Nutr. 2009, 139, 1839–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lamas, C.; de Castro, M.J.; Gil-Campos, M.; Gil, Á.; Couce, M.L.; Leis, R. Effects of dairy product consumption on height and bone mineral content in children: A systematic review of controlled trials. Adv. Nutr. 2019, 10, S88–S96. [Google Scholar] [CrossRef] [PubMed]
- Yackobovitch-Gavan, M.; Phillip, M.; Gat-Yablonski, G. How milk and its proteins affect growth, bone health, and weight. Horm. Res. Paediatr. 2017, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P. Dairy and Bone Health. J. Am. Coll. Nutr. 2009, 28, 82S–90S. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Kassem, M.; Møller, M.K.; Eriksen, E.F. The effects of IGF-I and IGF-II on proliferation and differentiation of human osteoblasts and interactions with growth hormone. Eur. J. Clin. Investig. 1998, 28, 176–183. [Google Scholar] [CrossRef]
- FitzGerald, R.J. Potential uses of caseinophosphopeptides. Int. Dairy J. 1998, 8, 451–457. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium, dairy products and osteoporosis. J. Am. Coll. Nutr. 2000, 19, 83S–99S. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, S.; Guo, B.; Miao, B.; Luo, Z.; Xia, Z.; Ying, D.; Liu, F.; Guo, B.; Tang, J.; et al. Bioactive peptide isolated from casein phosphopeptides promotes calcium uptake in vitro and in vivo. Food Funct. 2018, 9, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Masarwi, M.; Gabet, Y.; Dolkart, O.; Brosh, T.; Shamir, R.; Phillip, M.; Gat-Yablonski, G. Skeletal effect of casein and whey protein intake during catch-up growth in young male Sprague–Dawley rats. Br. J. Nutr. 2016, 116, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015, 101, 1330S–1338S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mølgaard, C.; Larnkjær, A.; Arnberg, K.; Michaelsen, K.F. Milk and growth in children: Effects of whey and casein. In Nestlé Nutrition Institute Workshop Series; Clemens, R.A., Hernell, O., Michaelsen, K.F., Eds.; Nestec Ltd.: Vevey, Switzerland, 2011; Volume 67, pp. 67–78. ISBN 1664-2147. [Google Scholar]
- WHO. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- CREA. Linee Guida per una Sana Alimentazione; Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria: Rome, Italy, 2018.
- Farr, J.N.; Dimitri, P. The Impact of Fat and Obesity on Bone Microarchitecture and Strength in Children. Calcif. Tissue Int. 2017, 100, 500–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, N.K. Childhood obesity, bone development, and cardiometabolic risk factors. Mol. Cell. Endocrinol. 2015, 410, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N.; Ghrelin, C.C.K. GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [Green Version]
- Morell, P.; Fiszman, S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocoll. 2017, 68, 199–210. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain: A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Kondrashina, A.; Brodkorb, A.; Giblin, L. Dairy-derived peptides for satiety. J. Funct. Foods 2020, 66, 103801. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef]
- Ferraretto, A.; Bottani, M.; De Luca, P.; Cornaghi, L.; Arnaboldi, F.; Maggioni, M.; Fiorilli, A.; Donetti, E. Morphofunctional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 2018, 38, BSR20171497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization. Standard ISO 8968-1:2014 [IDF 20-1:2014]. Milk and Milk Products—Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- International Organization for Standardization. Standard ISO 22662:2007 [IDF 198:2007]. Milk and Milk Products—Determination of Lactose Content by High-Performance Liquid Chromatography; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- International Organization for Standardization. Standard ISO 21415-1:2006. Wheat and Wheat Flour—Gluten Content—Part 1: Determination of Wet Gluten by a Manual Method; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef] [PubMed]
- McClean, P.; Weaver, L.T. Ontogeny of human pancreatic exocrine function. Arch. Dis. Child. 1993, 68, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekmati, M.; Ben-Shaul, Y.; Polak-Charcon, S. A morphological study of a human adenocarcinoma cell line (HT29) differentiating in culture. Similarities to intestinal embryonic development. Cell Differ. Dev. 1990, 31, 207–218. [Google Scholar] [CrossRef]
- Ferraretto, A.; Gravaghi, C.; Donetti, E.; Cosentino, S.; Donida, B.M.; Bedoni, M.; Lombardi, G.; Fiorilli, A.; Tettamanti, G. New methodological approach to induce a differentiation phenotype in Caco-2 cells prior to post-confluence stage. Anticancer Res. 2007, 27, 3919–3925. [Google Scholar]
- De Luca, P.; Bruschi, S.; Maggioni, M.; Stuknytė, M.; Cattaneo, S.; Bottani, M.; Fiorilli, A.; Rossi, F.; De Noni, I.; Ferraretto, A. Gastrointestinal digestates of Grana Padano and Trentingrana cheeses promote intestinal calcium uptake and extracellular bone matrix formation in vitro. Food Res. Int. 2016, 89, 820–827. [Google Scholar] [CrossRef]
- Maggioni, M.; Stuknytė, M.; De Luca, P.; Cattaneo, S.; Fiorilli, A.; De Noni, I.; Ferraretto, A. Transport of wheat gluten exorphins A5 and C5 through an in vitro model of intestinal epithelium. Food Res. Int. 2016, 88, 319–326. [Google Scholar] [CrossRef]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bottani, M.; Cornaghi, L.; Donetti, E.; Ferraretto, A. Excess of nutrient-induced morphofunctional adaptation and inflammation degree in a Caco2/HT-29 in vitro intestinal co-culture. Nutrition 2019, 58, 156–166. [Google Scholar] [CrossRef]
- Buras, R.R.; Shabahang, M.; Davoodi, F.; Schumaker, L.M.; Cullen, K.J.; Byers, S.; Nauta, R.J.; Evans, S.R.T. The effect of extracellular calcium on colonocytes: Evidence for differential responsiveness based upon degree of cell differentiation. Cell Prolif. 1995, 28, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Randall, R.J.; Farr, A.L. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Valli, V.; Danesi, F.; Gianotti, A.; Di Nunzio, M.; Taneyo Saa, D.L.; Bordoni, A. Antioxidative and anti-inflammatory effect of in vitro digested cookies baked using different types of flours and fermentation methods. Food Res. Int. 2016, 88, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Vitali, D.; Dragojević, I.V.; Šebečić, B. Effects of incorporation of integral raw materials and dietary fibre on the selected nutritional and functional properties of biscuits. Food Chem. 2009, 114, 1462–1469. [Google Scholar] [CrossRef]
- Obbagy, J.E.; English, L.K.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; Stoody, E.E. Complementary feeding and bone health: A systematic review. Am. J. Clin. Nutr. 2019, 109, 872S–878S. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Struijs, M.-C.; Diamond, I.R.; de Silva, N.; Wales, P.W. Establishing norms for intestinal length in children. J. Pediatr. Surg. 2009, 44, 933–938. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, F.A. Inflammatory Bowel Disease: Effects on bone and mechanisms. In Understanding the Gut-Bone Signaling Axis: Mechanisms and Therapeutic Implications; McCabe, L.R., Parameswaran, N., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 133–150. ISBN 978-3-319-66653-2. [Google Scholar]
- Zapata, R.C.; Singh, A.; Chelikani, P.K. Peptide YY mediates the satiety effects of diets enriched with whey protein fractions in male rats. FASEB J. 2018, 32, 850–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diepvens, K.; Häberer, D.; Westerterp-Plantenga, M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int. J. Obes. 2008, 32, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurrado, A.; Giungato, S.; Catacchio, I.; Piscitelli, D.; Arborea, G.; Piccinni, G.; Testini, M.; Vacca, A. Jejunal overexpression of peptide YY in celiac disease complicated with pneumatosis cystoides intestinalis. Clin. Exp. Med. 2015, 15, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Radavelli-Bagatini, S.; Hagger, M.; Ellis, V. Comparative effects of whey and casein proteins on satiety in overweight and obese individuals: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, A.P.; Sei, Y.; Zhao, X.; Feng, J.; Lu, X.; Thomas, C.; Pechhold, S.; Raybould, H.E.; Wank, S.A. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G538–G546. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.M.; Sun, E.W.; Keating, D.J. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J. Endocrinol. 2019, 244, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- De Bruïne, A.P.; Dinjens, W.N.; Pijls, M.M.; vd Linden, E.P.; Rousch, M.J.; Moerkerk, P.T.; de Goeij, A.F.; Bosnian, F.T. NCI-H716 cells as a model for endocrine differentiation in colorectal cancer. Virchows Arch. B 1992, 62, 311. [Google Scholar] [CrossRef]
- Brubaker, P.L.; Schloos, J.; Drucker, D.J. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line**This work was supported by grants from the Canadian Diabetes Association (Margaret A. Mollet grant, to P.L.B.) and Eli Lilly. Endocrinology 1998, 139, 4108–4114. [Google Scholar] [CrossRef]
- Foltz, M.; Ansems, P.; Schwarz, J.; Tasker, M.C.; Lourbakos, A.; Gerhardt, C.C. Protein hydrolysates induce CCK release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. J. Agric. Food Chem. 2008, 56, 837–843. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, T.E.L.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R. Effect of whey protein on the proliferation and differentiation of osteoblasts. J. Dairy Sci. 2009, 92, 3014–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millward, D.J.; Layman, D.K.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef]





| Ingredients | IB0 | IB1 | IB2 | IB3 | IB4 | |
|---|---|---|---|---|---|---|
| Wheat flour | 58.9 | 43.0 | 43.0 | 43.0 | 0.0 | |
| Wheat starch | 0.0 | 14.0 | 14.0 | 14.0 | 45.6 | |
| Sucrose | 18.2 | 18.9 | 18.9 | 16.3 | 18.2 | |
| Olive oil | 5.7 | 5.9 | 5.9 | 5.9 | 5.7 | |
| Dairy powders | MPC | 0.0 | 1.9 | 0.0 | 0.0 | 7.4 |
| WPI | 0.0 | 0.0 | 1.7 | 0.0 | 0.0 | |
| SMP | 0.0 | 0.0 | 0.0 | 4.7 | 0.0 | |
| Leavening agent | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | |
| Water | 16.8 | 15.8 | 15.8 | 15.8 | 22.9 |
| IB0 | IB1 | IB2 | IB3 | IB4 | |
|---|---|---|---|---|---|
| Gluten | 5.9 | 4.3 | 4.3 | 4.3 | 0.0 |
| Casein | 0.0 | 1.3 | 0.0 | 1.3 | 4.7 |
| Whey proteins | 0.0 | 0.3 | 1.6 | 0.3 | 1.2 |
| Lactose | 0.0 | traces | 0.0 | 2.5 | traces |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottani, M.; Cattaneo, S.; Pica, V.; Stuknytė, M.; Gomarasca, M.; Lombardi, G.; Banfi, G.; Noni, I.D.; Ferraretto, A. Gastrointestinal In Vitro Digests of Infant Biscuits Formulated with Bovine Milk Proteins Positively Affect In Vitro Differentiation of Human Osteoblast-Like Cells. Foods 2020, 9, 1510. https://doi.org/10.3390/foods9101510
Bottani M, Cattaneo S, Pica V, Stuknytė M, Gomarasca M, Lombardi G, Banfi G, Noni ID, Ferraretto A. Gastrointestinal In Vitro Digests of Infant Biscuits Formulated with Bovine Milk Proteins Positively Affect In Vitro Differentiation of Human Osteoblast-Like Cells. Foods. 2020; 9(10):1510. https://doi.org/10.3390/foods9101510
Chicago/Turabian StyleBottani, Michela, Stefano Cattaneo, Valentina Pica, Milda Stuknytė, Marta Gomarasca, Giovanni Lombardi, Giuseppe Banfi, Ivano De Noni, and Anita Ferraretto. 2020. "Gastrointestinal In Vitro Digests of Infant Biscuits Formulated with Bovine Milk Proteins Positively Affect In Vitro Differentiation of Human Osteoblast-Like Cells" Foods 9, no. 10: 1510. https://doi.org/10.3390/foods9101510