Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Plant Material
2.3. Compositional Analysis
2.3.1. Proximate Composition and Energy
2.3.2. Free Sugars
2.3.3. Organic Acids
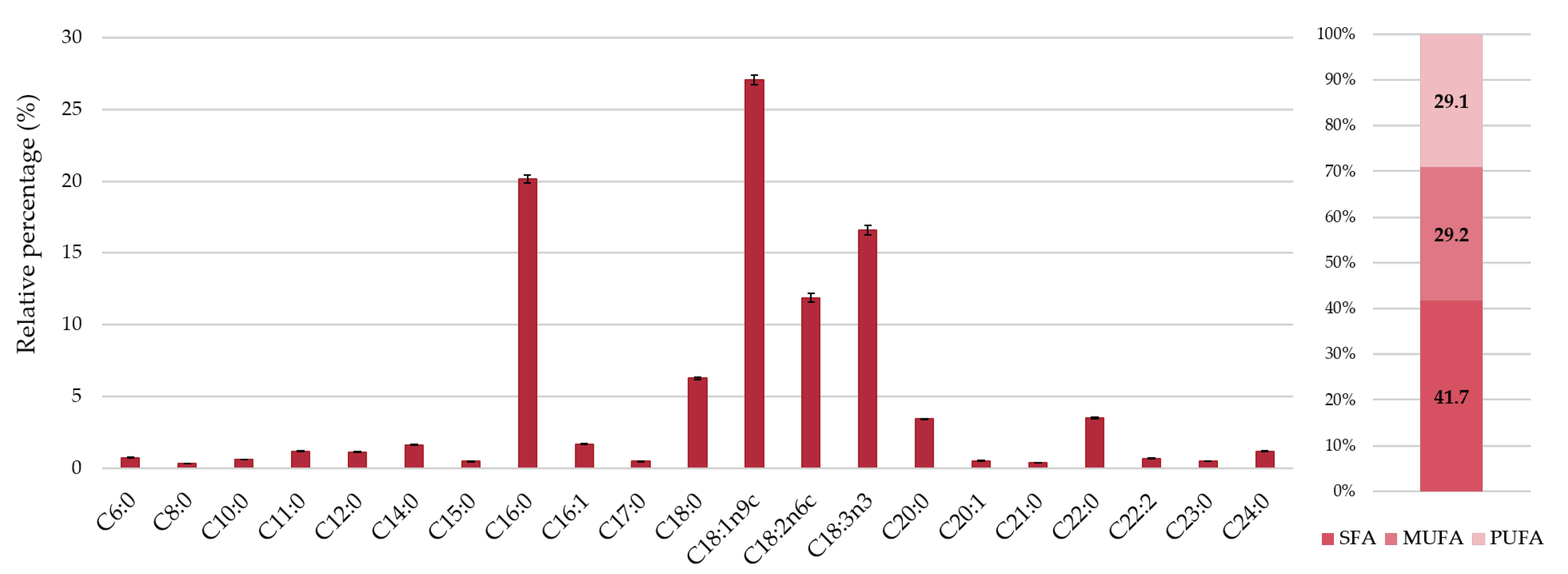
2.3.4. Fatty Acids
2.3.5. Tocopherols
2.4. Extract Preparation
2.5. Analysis of Anthocyanins
2.6. Evaluation of Bioactive Properties
2.6.1. Antioxidant Activity
2.6.2. Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.2. Anthocyanins Composition
3.3. Bioactive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schulz, M.; Chim, J.F. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 31. [Google Scholar] [CrossRef]
- Kim, M.J.; Sutton, K.L.; Harris, G.K. Raspberries and Related Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 586–591. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 8 March 2020).
- Overview Global Berry Market—FreshPlaza. Available online: https://www.freshplaza.com/article/9136082/overview-global-berry-market/ (accessed on 18 July 2020).
- Valdés García, A.; Maestre Pérez, S.E.; Butsko, M.; Prats Moya, M.S.; Beltrán Sanahuja, A. Authentication of “Adelita” raspberry cultivar based on physical properties, antioxidant activity and volatile profile. Antioxidants 2020, 9, 593. [Google Scholar]
- Andrianjaka-Camps, Z.N.; Wittemann, M.S.; Ançay, A.; Carlen, C. New cultivars for quality production of primocane fruiting raspberries enriched in healthy compounds. Acta Hortic. 2016, 1133, 345–352. [Google Scholar] [CrossRef]
- Advanced Berry Breeding—Kweli®. Available online: https://www.abbreeding.nl/varieties/kweli/?lang=en (accessed on 19 July 2020).
- Strik, B.C.; Moore, P.P. Raspberry Cultivars for the Pacific Northwest; Oregon State University: Corvallis, OR, USA, 2014. [Google Scholar]
- Hanson, E.; Crain, B.; Hanson, K. Response of potted red raspberry cultivars to double-cropping under high tunnels. HortScience 2019, 54, 1972–1975. [Google Scholar] [CrossRef] [Green Version]
- De Souza, V.R.; Pereira, P.A.P.; Da Silva, T.L.T.; De Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanov, D.; Milošević, T.; Mašković, P.; Milošević, N.; Glišić, I.; Paunović, G. Influence of organic, organo-mineral and mineral fertilisers on cane traits, productivity and berry quality of red raspberry (Rubus idaeus L.). Sci. Hortic. 2019, 252, 370–378. [Google Scholar] [CrossRef]
- Kafkas, E.; Özgen, M.; Özoğul, Y.; Türemiş, N. Phytochemical and fatty acid profile of selected red raspberry cultivars: A comparative study. J. Food Qual. 2008, 31, 67–78. [Google Scholar] [CrossRef]
- Çekiç, Ç.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compos. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef]
- Haffner, K.; Rosenfeld, H.J.; Skrede, G.; Wang, L. Quality of red raspberry Rubus idaeus L. cultivars after storage in controlled and normal atmospheres. Postharvest Biol. Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Giovanelli, G.; Limbo, S.; Buratti, S. Effects of new packaging solutions on physico-chemical, nutritional and aromatic characteristics of red raspberries (Rubus idaeus L.) in postharvest storage. Postharvest Biol. Technol. 2014, 98, 72–81. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; Van Der Meer, I.M.; De Vos, C.H.R. Antioxidants in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Anjos, R.; Cosme, F.; Gonçalves, A.; Nunes, F.M.; Vilela, A.; Pinto, T. Effect of agricultural practices, conventional vs organic, on the phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.). Food Chem. 2020, 328, 126833. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103568. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union 2011, 54. [Google Scholar]
- Pinela, J.; Barreira, J.C.M.; Barros, L.; Cabo Verde, S.; Antonio, A.L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Suitability of gamma irradiation for preserving fresh-cut watercress quality during cold storage. Food Chem. 2016, 206, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Iyda, J.H.; Fernandes, Â.; Ferreira, F.D.; Alves, M.J.; Pires, T.C.S.P.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 2019, 121, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Pinela, J.; Antonio, A.L.; Barros, L.; Barreira, J.C.M.; Carvalho, A.M.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Combined effects of gamma-irradiation and preparation method on antioxidant activity and phenolic composition of Tuberaria lignosa. RSC Adv. 2015, 5, 14756–14767. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [Green Version]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- INSA PortFIR—Plataforma Portuguesa de Informação Alimentar. Available online: http://portfir.insa.pt/foodcomp/food?14110 (accessed on 19 April 2020).
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; VerhÉ, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- USDA FoodData Central—Raspberries, Red, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/786783/nutrients (accessed on 13 July 2020).
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and antioxidant properties of cultivated and wild fragaria and Rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Bobinait, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- IOM (Institute of Medicine). Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Carvalho, E.; Fraser, P.D.; Martens, S. Carotenoids and tocopherols in yellow and red raspberries. Food Chem. 2013, 139, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.A.; Munné-Bosch, S. Abscisic acid and pyrabactin improve vitamin C contents in raspberries. Food Chem. 2016, 203, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Peter Yurawecz, M.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.R.; Jennings, M.H.; Rome, C.; Hadjivassiliou, V.; Papas, K.A.; Alexander, J.S. α-, γ- and δ-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J. Nutr. Biochem. 2010, 21, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Caidan, R.; Cairang, L.; Liubin; Yourui, S. Simultaneous analysis of fatty acids in Rubus niveus Thunb. fruits by HPLC-MS/MS. Asian J. Chem. 2013, 25, 1866–1870. [Google Scholar] [CrossRef]
- Ferreyra, R.; Sellés, G.; Saavedra, J.; Ortiz, J.; Zúñiga, C.; Troncoso, C.; Rivera, S.A.; González-Agüero, M.; Defilippi, B.G. Identification of pre-harvest factors that affect fatty acid profiles of avocado fruit (Persea americana Mill) cv. “Hass” at harvest. S. Afr. J. Bot. 2016, 104, 15–20. [Google Scholar] [CrossRef]
- Mapiye, C.; Chimonyo, M.; Dzama, K.; Hugo, A.; Strydom, P.E.; Muchenje, V. Fatty acid composition of beef from Nguni steers supplemented with Acacia karroo leaf-meal. J. Food Compos. Anal. 2011, 24, 523–528. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Kula, M.; Majdan, M.; Głód, D.; Krauze-Baranowska, M. Phenolic composition of fruits from different cultivars of red and black raspberries grown in Poland. J. Food Compos. Anal. 2016, 52, 74–82. [Google Scholar] [CrossRef]
- Lee, J.; Dossett, M.; Finn, C.E. Rubus fruit phenolic research: The good, the bad, and the confusing. Food Chem. 2012, 130, 785–796. [Google Scholar] [CrossRef]
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Gião, M.S.; Leitão, I.; Pereira, A.; Borges, A.B.; Guedes, C.J.; Fernandes, J.C.; Belo, L.; Santos-Silva, A.; Hogg, T.A.; Pintado, M.E.; et al. Plant aqueous extracts: Antioxidant capacity via haemolysis and bacteriophage P22 protection. Food Control 2010, 21, 633–638. [Google Scholar] [CrossRef]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Constituent | Content (fw) | Content (dw) |
|---|---|---|
| Moisture (g/100 g) 1 | 83.1 ± 0.2 | - |
| Proteins (g/100 g) | 0.18 ± 0.01 | 6.8 ± 0.4 |
| Ash (g/100 g) | 0.66 ± 0.02 | 3.90 ± 0.09 |
| Fat (g/100 g) | 0.132 ± 0.005 | 0.78 ± 0.03 |
| Total carbohydrates (g/100 g) | 16.12 ± 0.01 | 88.5 ± 0.3 |
| Energy (kcal/100 g) | 66.40 ± 0.04 | 388 ± 1 |
| Fructose (g/100 g) | 2.42 ± 0.03 | 14.3 ± 0.2 |
| Glucose (g/100 g) | 2.13 ± 0.09 | 12.6 ± 0.5 |
| Sucrose (g/100 g) | 1.41 ± 0.02 | 8.3 ± 0.1 |
| Trehalose (g/100 g) | 0.020 ± 0.001 | 0.140 ± 0.008 |
| Raffinose (g/100 g) | 0.040 ± 0.001 | 0.260 ± 0.002 |
| Total sugars (g/100 g) | 6.0 ± 0.1 | 35.7 ± 0.8 |
| Ascorbic acid (mg/100 g) | 17 ± 1 | 100 ± 7 |
| Citric acid (mg/100 g) | 2718 ± 134 | 16066 ± 790 |
| Fumaric acid (mg/100 g) | 1.5 ± 0.1 | 8.9 ± 0.6 |
| Total organic acids (mg/100 g) | 2765 ± 136 | 16175 ± 798 |
| α-Tocopherol (mg/100 g) | 0.050 ± 0.001 | 0.29 ± 0.01 |
| γ-Tocopherol (mg/100 g) | 0.52 ± 0.02 | 3.1 ± 0.01 |
| δ-Tocopherol (mg/100 g) | 1.36 ± 0.03 | 8.0 ± 0.2 |
| Total tocopherols (mg/100 g) | 1.92 ± 0.05 | 11.4 ± 0.3 |
| Peak | Rt (min) | λmax (nm) | [M − H]+ (m/z) | MS2 Fragments (m/z) | Tentative Identification | Content (mg/g Extract) |
|---|---|---|---|---|---|---|
| 1 | 11.48 | 515 | 611 | 287 (100) | Cyanidin-O-sophoroside | 2.82 ± 0.03 |
| 2 | 14.26 | 512 | 449 | 287 (100) | Cyanidin-O-hexoside | 1.69 ± 0.02 |
| Total anthocyanins | 4.51 ± 0.04 |
| Bioactivity | Red Raspberry Extract | Positive Controls | ||||
|---|---|---|---|---|---|---|
| Antioxidant activity | Trolox | |||||
| TBARS assay (EC50, µg/mL) | 122 ± 2 | 5.4 ± 0.3 | ||||
| OxHLIA assay (IC50, µg/mL) | 298 ± 17 | 19 ± 1 | ||||
| β-CBI assay (EC50, µg/mL) | 18.7 ± 0.2 | 0.20 ± 0.02 | ||||
| E211 | E224 | |||||
| Antibacterial activity | MIC | MBC | MIC | MBC | MIC | MBC |
| Bacillus cereus | 0.78 | 1.56 | 0.5 | 0.5 | 2 | 4 |
| Listeria monocytogenes | 3.12 | 6.24 | 1 | 2 | 0.5 | 1 |
| Escherichia coli | 3.12 | 6.24 | 1 | 2 | 0.5 | 1 |
| Salmonella typhimurium | 3.12 | 6.24 | 1 | 2 | 1 | 1 |
| Antifungal activity | MIC | MFC | MIC | MFC | MIC | MFC |
| Aspergillus fumigatus | >6.24 | >6.24 | 1 | 2 | 1 | 1 |
| Aspergillus niger | 3.12 | 6.24 | 1 | 2 | 1 | 1 |
| Penicillium verrucosum var. cyclopium | >6.24 | >6.24 | 2 | 4 | 1 | 1 |
| Trichoderma viride | 1.56 | 3.12 | 1 | 2 | 0.5 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vara, A.L.; Pinela, J.; Dias, M.I.; Petrović, J.; Nogueira, A.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities. Foods 2020, 9, 1522. https://doi.org/10.3390/foods9111522
Vara AL, Pinela J, Dias MI, Petrović J, Nogueira A, Soković M, Ferreira ICFR, Barros L. Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities. Foods. 2020; 9(11):1522. https://doi.org/10.3390/foods9111522
Chicago/Turabian StyleVara, Ana Luísa, José Pinela, Maria Inês Dias, Jovana Petrović, António Nogueira, Marina Soković, Isabel C. F. R. Ferreira, and Lillian Barros. 2020. "Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities" Foods 9, no. 11: 1522. https://doi.org/10.3390/foods9111522
APA StyleVara, A. L., Pinela, J., Dias, M. I., Petrović, J., Nogueira, A., Soković, M., Ferreira, I. C. F. R., & Barros, L. (2020). Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities. Foods, 9(11), 1522. https://doi.org/10.3390/foods9111522











