Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Winemaking and Bentonite Treatments
2.1.1. Experiment 1: Effect of the Moment of Bentonite Addition
2.1.2. Experiment 2: Effect of Co-Addition of Enological Tannins
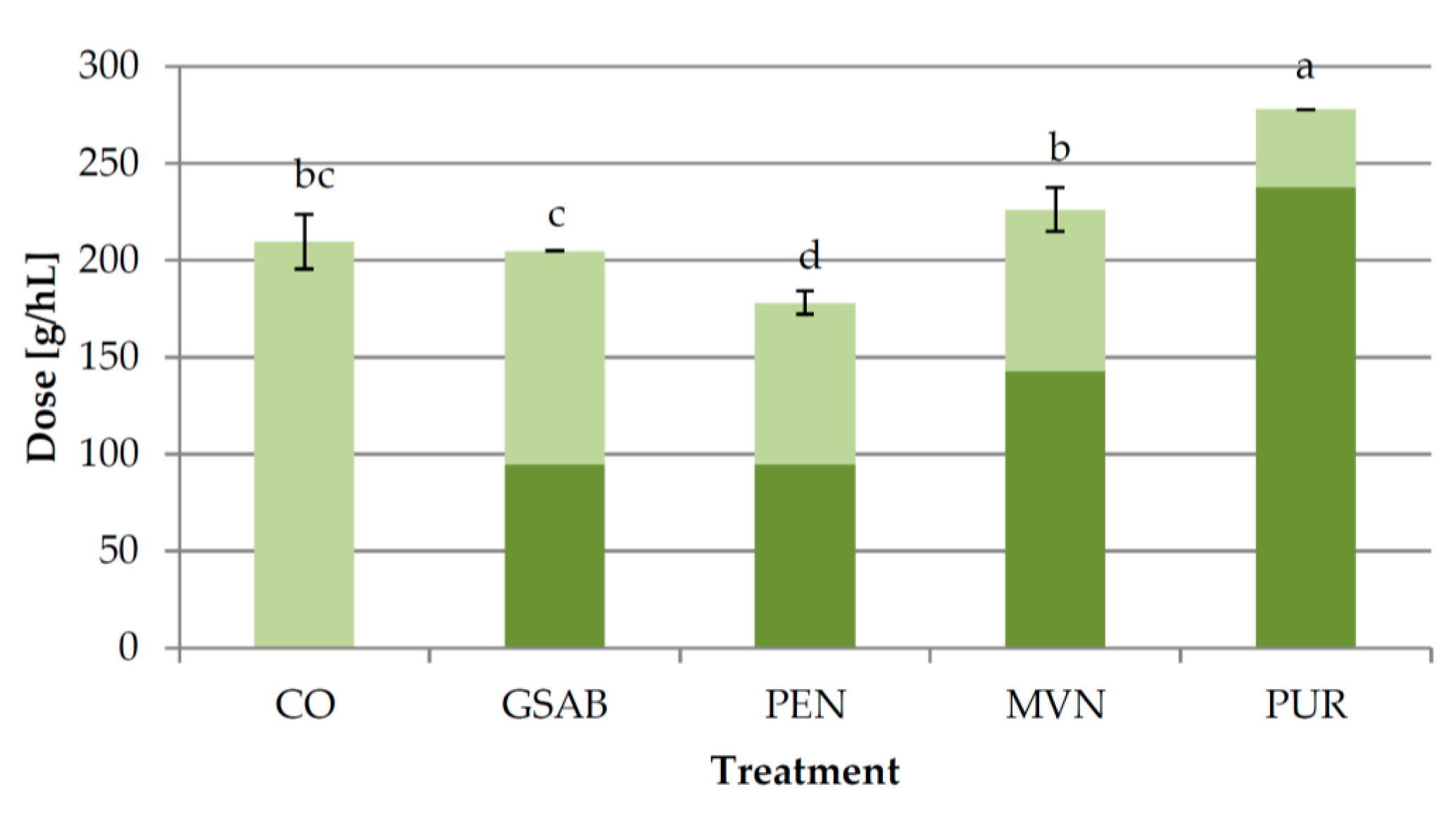
2.1.3. Experiment 3: Effect of Bentonite Type
2.2. Post-Fermentation Practices
2.3. Protein Stability Tests
2.4. Analysis of Pathogenesis-Related (PR) Proteins by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.5. Analysis of Proteins by Size Exclusion High-Performance Liquid Chromatography (SE-HPLC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1: Effect of the Moment of Bentonite Addition
3.2. Experiment 2: Effect of Co-Addition of Enological Tannins
3.3. Experiment 3: Effect of Bentonite Type
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marangon, M.; Stockdale, V.J.; Munro, P.; Trethewey, T.; Schulkin, A.; Holt, H.E.; Smith, P.A. Addition of Carrageenan at Different Stages of Winemaking for White Wine Protein Stabilization. J. Agric. Food Chem. 2013, 61, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.K.; Muthukrishnan, S. Pathogenesis-Related Proteins in Plants, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-0697-6. [Google Scholar]
- Marangon, M.; Lucchetta, M.; Waters, E.J. Protein stabilisation of white wines using zirconium dioxide enclosed in a metallic cage. Aust. J. Grape Wine Res. 2011, 17, 28–35. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Characterization of natural haze protein in Sauvignon white wine. Food Chem. 2009, 113, 28–35. [Google Scholar] [CrossRef]
- Sauvage, F.-X.; Bach, B.; Moutounet, M.; Vernhet, A. Proteins in white wines: Thermo-sensitivity and differential adsorbtion by bentonite. Food Chem. 2010, 118, 26–34. [Google Scholar] [CrossRef]
- Falconer, R.J.; Marangon, M.; Van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Waters, E.J. Thermal stability of thaumatin-like protein, chitinase, and invertase isolated from Sauvignon blanc and Semillon juice and their role in haze formation in wine. J. Agric. Food Chem. 2010, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.; Waters, E.J.; Herderich, M.J.; Pretorius, I.S. Recent advances help us understand protein haze more clearly. Aust. N. Z. Wine Ind. J. 2010, 25, 24–27. [Google Scholar]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Høj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of Bentonite Fining on Odor-Active Compounds in Two Different White Wine Styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar]
- Majewski, P.; Barbalet, A.; Waters, E. $1 billion hidden cost of bentonite fining. Aust. N. Z. Grapegrow. Winemak. 2011, 569, 58–62. [Google Scholar]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Dordoni, R.; Colangelo, D.; Giribaldi, M.; Giuffrida, M.G.; De Faveri, D.M.; Lambri, M. Effect of Bentonite Characteristics on Wine Proteins, Polyphenols, and Metals under Conditions of Different pH. Am. J. Enol. Vitic. 2015, 66, 518–530. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Comparing the impact of bentonite addition for both must clarification and wine fining on the chemical profile of wine from Chambave Muscat grapes. Int. J. Food Sci. Tech. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aromas, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and Wine Proteins: Their Fractionation by Hydrophobic Interaction Chromatography and Identification by Chromatographic and Proteomic Analysis. J. Agric. Food Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-Bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: Its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res. Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.C.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Pocock, K.F.; Høj, P.B.; Adams, K.S.; Kwiatkowski, M.J.; Waters, E.J. Combined heat and proteolytic enzyme treatment of white wines reduces haze forming protein content without detrimental effect. Aust. J. Grape Wine Res. 2003, 9, 56–63. [Google Scholar] [CrossRef]
- Pashova, V.; Güell, C.; Pueyo, E.; López-Barajas, M.; Polo, M.C.; López, F. White wine protein stabilization by a continuous process using a packed column. Am. J. Enol. Vitic. 2004, 55, 195–198. [Google Scholar]
- Lucchetta, M.; Pocock, K.F.; Waters, E.J.; Marangon, M. Use of Zirconium Dioxide during Fermentation as an Alternative to Protein Fining with Bentonite for White Wines. Am. J. Enol. Vitic. 2013, 64, 400–404. [Google Scholar] [CrossRef]
- Salazar, F.N.; Achaerandio, I.; Labbé, M.A.; Güell, C.; López, F. Comparative study of protein stabilization in white wine using zirconia and bentonite: Physicochemical and wine sensory analysis. J. Agric. Food Chem. 2006, 54, 9955–9958. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Lucchetta, M.; Duan, D.; Stockdale, V.J.; Hart, A.; Rogers, P.J.; Waters, E.J. Protein removal from a Chardonnay juice by addition of carrageenan and pectin. Aust. J. Grape Wine Res. 2012, 18, 194–202. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Mierczynski, P.; Maniukiewicz, W.; Visalakshan, R.M.; Vasilev, K.; Smith, P.A. Magnetic separation technology: Functional group efficiency in the removal of haze-forming proteins from wines. Food Chem. 2019, 275, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Wahono, S.K.; Smith, P.A.; Bindon, K.; Vasilev, K. Using Zeolites To Protein Stabilize White Wines. ACS Sustain. Chem. Eng. 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Muhlack, R.; Nordestgaard, S.; Waters, E.J.; O’Neill, B.K.; Lim, A.; Colby, C.B. In-line dosing for bentonite fining of wine or juice: Contact time, clarification, product recovery and sensory effects. Aust. J. Grape Wine Res. 2006, 12, 221–234. [Google Scholar] [CrossRef]
- Lira, E.; Rodríguez-Bencomo, J.J.; Salazar, F.N.; Orriols, I.; Fornos, D.; López, F. Impact of Bentonite Additions during Vinification on Protein Stability and Volatile Compounds of Albariño Wines. J. Agric. Food Chem. 2015, 63, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int. J. Food Sci. Tech. 2014, 49, 1070–1078. [Google Scholar] [CrossRef]
- Pocock, K.F.; Salazar, F.N.; Waters, E.J. The effect of bentonite fining at different stages of white winemaking on protein stability. Aust. J. Grape Wine Res. 2011, 17, 280–284. [Google Scholar] [CrossRef]
- Jaeckels, N.; Tenzer, S.; Meier, M.; Will, F.; Dietrich, H.; Decker, H.; Fronk, P. Influence of bentonite fining on protein composition in wine. Lwt-Food Sci. Technol. 2017, 75, 335–343. [Google Scholar] [CrossRef]
- Monteiro, S.; Piçarra-Pereira, M.A.; Mesquita, P.R.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The wide diversity of structurally similar wine proteins. J. Agric. Food Chem. 2001, 49, 3999–4010. [Google Scholar] [CrossRef]
- Larcher, R.; Tonidandel, L.; Villegas, T.R.; Nardin, T.; Fedrizzi, B.; Nicolini, G. Pre-Fermentation Addition Of Grape Tannin Increases The Varietal Thiols Content In Wine. Food Chem. 2015, 166, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sonni, F.; Cejudo Bastante, M.J.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- Sonni, F.; Chinnici, F.; Natali, N.; Riponi, C. Pre-fermentative replacement of sulphur dioxide by lysozyme and oenological tannins: Effect on the formation and evolution of volatile compounds during the bottle storage of white wines. Food Chem. 2011, 129, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E. Chemistry of tannin-protein complexation. In Chemistry and Significance of Condensed Tannins, 1st ed.; Hemingway, R.W., Karchesy, J.J., Eds.; Plenum Press: New York, NY, USA, 1989; pp. 323–331. ISBN 978-1-4684-7513-5. [Google Scholar]
- Haslam, E.; Lilley, T.H.; Warminski, E.; Liao, H.; Cai, Y.; Martin, R.; Gaffney, S.H.; Goulding, P.N.; Luck, G. Polyphenol complexation. A study in molecular recognition. In Phenolic Compounds in Food and Their Effects on Health I: Analysis, Ocurrence, & Chemistry, 1st ed.; Ho, C.-T., Lee, C.Y., Huang, M.-T., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 8–50. ISBN 978-0-8412-2475-9. [Google Scholar]
- Terrier, N.; Poncet-Legrand, C.; Cheynier, V. Flavanols, Flavonols and Dihydroflavonols. In Wine Chemistry and Biochemistry, 1st ed.; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 463–507. ISBN 978-0-387-74116-1. [Google Scholar]
- Powers, J.R.; Nagel, C.W.; Weller, K. Protein Removal from a Wine by Immobilized Grape Proanthocyanidins. Am. J. Enol. Vitic. 1988, 39, 117–120. [Google Scholar]
- Radeka, S.; Peršurić, Đ.; Lukić, I.; Bocca, E.; Plavša, T. Influence of the addition of tannins of different origin on protein stability and aromatic profile of Malvazija istarska wine. In Proceedings of the 32nd World Congress of Vine and Wine and 7th General Assembly of the OIV, Zagreb, Croatia, 28 June–3 July 2009; Kurbanovic, V., Ed.; Ministry of Agriculture, Fisheries and Rural Development: Zagreb, Croatia, 2009. [Google Scholar]
- Van Sluyter, S.C.; Marangon, M.; Stranks, S.D.; Neilson, K.A.; Hayasaka, Y.; Haynes, P.A.; Menz, R.I.; Waters, E.J. Two-step purification of pathogenesis-related proteins from grape juice and crystallization of thaumatin-like proteins. J. Agric. Food Chem. 2009, 57, 11376–11382. [Google Scholar] [CrossRef]
- Pashova, V.; Güell, C.; López, F. White Wine Continuous Protein Stabilization by Packed Column. J. Agric. Food Chem. 2004, 52, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Le Bourse, D.; Conreux, A.; Villaume, S.; Lameiras, P.; Nuzillard, J.-M.; Jeandet, P. Quantification of chitinase and thaumatin-like proteins in grape juices and wines. Anal. Bioanal. Chem. 2011, 401, 1541–1549. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Tech. 2002, 12, 230–239. [Google Scholar] [CrossRef]
- Dordoni, R.; Galasi, R.; Colangelo, D.; De Faveri, D.M.; Lambri, M. Effects of fining with different bentonite labels and doses on colloidal stability and colour of a Valpolicella red wine. Int. J. Food Sci. Tech. 2015, 50, 2246–2254. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef]
- Gazzola, D.; Van Sluyter, S.C.; Curioni, A.; Waters, E.J.; Marangon, M. Roles of Proteins, Polysaccharides, and Phenolics in Haze Formation in White Wine via Reconstitution Experiments. J. Agric. Food Chem. 2012, 60, 10666–10673. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, C.; Wu, Z.; Xu, X.; Ren, L.; Zhao, H. Adsorption of Protein from Model Wine Solution by Different Bentonites. Chin. J. Chem. Eng. 2007, 15, 632–638. [Google Scholar] [CrossRef]
- Blade, W.H.; Boulton, R. Adsorption of Protein by Bentonite in a Model Wine Solution. Am. J. Enol. Vitic. 1988, 39, 193–199. [Google Scholar]
- Achaerandio, I.; Pachova, V.; Güell, C.; López, F. Protein Adsorption by Bentonite in a White Wine Model Solution: Effect of Protein Molecular Weight and Ethanol Concentration. Am. J. Enol. Vitic. 2001, 52, 122–126. [Google Scholar]
- De Bruijn, J.; Loyola, C.; Flores, A.; Hevia, F.; Melìn, P.; Serra, I. Protein stabilisation of Chardonnay wine using trisacryl and bentonite: A comparative study. Int. J. Food Sci. Tech. 2009, 44, 330–336. [Google Scholar] [CrossRef]
- Sommer, S.; Wegmann-Herr, P.; Fischer, U. Correlating the need for bentonite fining in wine with anomalous weather patterns. J. Wine Res. 2015, 26, 29–39. [Google Scholar] [CrossRef]





| Protein | Stage | Treatment | ||||
|---|---|---|---|---|---|---|
| CO | JU | BE | MD | EN | ||
| RP-HPLC1 | ||||||
| TL1 | AFerm | 54.45 ± 1.54 a | 26.04 ± 3.80 b | 28.41 ± 11.30 b | 23.91 ± 3.52 b | 17.45 ± 4.88 b |
| ProStab | 1.37 ± 0.37 a | 0.74 ± 0.11 b | 0.66 ± 0.05 b | 0.48 ± 0.26 b | 0.69 ± 0.13 b | |
| TL2 | AFerm | 22.85 ± 0.19 a | 15.47 ± 0.65 b | 15.18 ± 1.59 b | 14.8 ± 0.47 b | 10.85 ± 2.36 c |
| ProStab | 3.24 ± 0.80 a | 1.63 ± 0.57 b | 1.53 ± 0.21 b | 1.55 ± 0.10 b | 2.41 ± 0.17 a | |
| TL3 | AFerm | 19.17 ± 0.54 a | 15.00 ± 0.85 ab | 15.55 ± 2.60 ab | 16.4 ± 0.96 ab | 12.96 ± 3.14 b |
| ProStab | 6.91 ± 1.74 a | 2.39 ± 0.94 c | 2.52 ± 0.47 c | 2.74 ± 0.33 c | 4.22 ± 0.36 b | |
| TL4 | AFerm | 67.76 ± 0.80 a | 27.87 ± 3.55 b | 26.49 ± 4.13 b | 8.34 ± 1.46 c | 2.30 ± 1.01 d |
| ProStab | 0.97 ± 0.63 | 0.72 ± 0.04 | 0.58 ± 0.12 | 0.81 ± 0.17 | 0.77 ± 0.07 | |
| CHI1 | AFerm | 73.66 ± 5.81 a | 38.1 ± 9.79 b | 41.74 ± 12.35 b | 40.84 ± 6.80 b | 32.72 ± 6.09 b |
| ProStab | 0.95 ± 0.01 | 0.64 ± 0.21 | 0.87 ± 0.06 | 0.91 ± 0.03 | 0.84 ± 0.07 | |
| CHI2 | AFerm | 57.23 ± 2.47 a | 29.89 ± 6.50 b | 30.31 ± 8.86 b | 29.27 ± 5.26 b | 21.82 ± 4.84 b |
| ProStab | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.15 ± 0.01 | 0.17 ± 0.06 | 0.16 ± 0.02 | |
| SE-HPLC2 | ||||||
| P93 | AFerm | 13.89 ± 0.56 a | 10.19 ± 0.12 c | 10.41 ± 1.08 bc | 11.30 ± 1.03 bc | 11.98 ± 0.66 b |
| ProStab | 11.00 ± 0.57 | 9.58 ± 0.54 | 9.94 ± 1.23 | 9.84 ± 0.98 | 11.22 ± 0.92 | |
| P67 | AFerm | 13.27 ± 1.81 a | 7.38 ± 0.92 b | 5.97 ± 0.98 b | 7.15 ± 0.39 b | 6.12 ± 1.13 b |
| ProStab | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PR32 | AFerm | 4.30 ± 1.66 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| ProStab | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PR25 | AFerm | 228.15 ± 3.64 a | 119.85 ± 10.56 b | 103.85 ± 1.02 b | 107.91 ± 3.28 b | 101.61 ± 16.13 b |
| ProStab | 26.20 ± 8.06 a | 5.01 ± 1.97 b | 5.00 ± 0.29 b | 4.51 ± 1.18 b | 8.62 ± 0.68 b | |
| PR23 | AFerm | 90.07 ± 2.11 a | 61.95 ± 5.76 b | 48.47 ± 0.49 cd | 52.84 ± 0.63 c | 43.33 ± 6.96 d |
| ProStab | 9.36 ± 3.89 a | 2.03 ± 1.09 b | 1.58 ± 0.04 b | 1.74 ± 0.49 b | 4.00 ± 0.55 b | |
| PR22 | AFerm | 135.15 ± 3.32 a | 56.08 ± 6.87 b | 52.19 ± 6.46 b | 35.39 ± 2.76 c | 20.41 ± 3.83 d |
| ProStab | 8.23 ± 3.23 a | 1.97 ± 0.85 b | 2.09 ± 0.03 b | 1.71 ± 0.44 b | 2.84 ± 0.30 b | |
| PR20 | AFerm | 97.54 ± 7.11 a | 45.31 ± 0.71 b | 40.60 ± 1.86 b | 31.42 ± 4.28 c | 25.02 ± 2.00 c |
| ProStab | 14.81 ± 0.41 a | 9.34 ± 0.32 b | 10.18 ± 0.93 b | 7.89 ± 1.09 c | 7.48 ± 0.52 c | |
| Protein | Stage | Treatment | |||
|---|---|---|---|---|---|
| CO | GSAB | ET | GSAB + ET | ||
| RP-HPLC1 | |||||
| TL1 | AFerm | 38.11 ± 6.14 a | 8.31 ± 1.71 c | 29.03 ± 3.50 b | 4.00 ± 1.34 c |
| ProStab | 1.40 ± 1.78 | 1.05 ± 0.89 | 1.41 ± 0.94 | 0.29 ± 0.07 | |
| TL2 | AFerm | 13.87 ± 1.16 a | 5.48 ± 0.41 c | 11.34 ± 1.13 b | 3.97 ± 0.92 c |
| ProStab | 1.89 ± 0.67 | 1.89 ± 0.48 | 1.66 ± 0.02 | 1.68 ± 0.20 | |
| TL3 | AFerm | 13.94 ± 0.69 a | 9.61 ± 0.26 b | 12.37 ± 2.06 a | 9.76 ± 1.35 b |
| ProStab | 3.85 ± 0.97 | 4.90 ± 0.83 | 3.46 ± 0.25 | 4.23 ± 0.54 | |
| TL4 | AFerm | 30.17 ± 3.17 a | 0.73 ± 0.53 c | 24.16 ± 1.69 b | 0.29 ± 0.19 c |
| ProStab | 0.70 ± 0.08 | 0.00 ± 0.00 | 1.21 ± 0.86 | 0.06 ± 0.10 | |
| CHI1 | AFerm | 36.63 ± 3.38 a | 17.42 ± 2.17 b | 34.66 ± 5.58 a | 10.49 ± 1.27 c |
| ProStab | 3.49 ± 2.44 | 4.74 ± 2.09 | 3.95 ± 2.05 | 2.14 ± 1.00 | |
| CHI2 | AFerm | 31.45 ± 3.27 a | 14.44 ± 2.25 b | 31.62 ± 5.30 a | 8.55 ± 0.94 b |
| ProStab | 2.80 ± 1.63 | 4.28 ± 1.59 | 3.75 ± 1.87 | 1.82 ± 0.76 | |
| SE-HPLC2 | |||||
| P93 | AFerm | 8.38 ± 0.84 | 8.05 ± 0.03 | 7.12 ± 1.11 | 7.26 ± 1.32 |
| ProStab | 8.88 ± 0.74 | 7.86 ± 0.20 | 9.04 ± 0.95 | 8.91 ± 0.11 | |
| P67 | AFerm | 7.55 ± 0.63 a | 4.64 ± 0.12 bc | 4.85 ± 0.46 b | 3.00 ± 1.59 c |
| ProStab | 0.90 ± 0.85 | 0.90 ± 0.35 | 0.32 ± 0.55 | 0.00 ± 0.00 | |
| PR32 | AFerm | 5.13 ± 0.42 a | 0.00 ± 0.00 c | 3.63 ± 0.45 b | 0.00 ± 0.00 c |
| ProStab | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PR25 | AFerm | 94.65 ± 5.64 a | 44.94 ± 5.49 c | 71.52 ± 1.25 b | 24.99 ± 2.58 d |
| ProStab | 11.09 ± 7.56 | 12.77 ± 3.45 | 7.22 ± 2.13 | 7.27 ± 1.68 | |
| PR23 | AFerm | 55.60 ± 6.47 a | 21.51 ± 2.98 c | 38.99 ± 1.78 b | 10.36 ± 2.63 d |
| ProStab | 5.12 ± 3.79 | 4.39 ± 1.40 | 2.83 ± 1.32 | 2.53 ± 0.92 | |
| PR22 | AFerm | 41.51 ± 2.38 a | 7.40 ± 1.12 c | 34.47 ± 2.32 b | 3.01 ± 1.12 d |
| ProStab | 3.33 ± 2.05 a | 1.19 ± 0.50 b | 0.38 ± 0.48 b | 0.18 ± 0.16 b | |
| PR20 | AFerm | 58.04 ± 4.45 a | 14.09 ± 0.69 c | 45.23 ± 2.32 b | 11.67 ± 2.36 c |
| ProStab | 10.69 ± 1.50 a | 6.84 ± 0.40 c | 7.38 ± 0.56 bc | 9.19 ± 1.02 ab | |
| Protein | Stage | Treatment | ||||
|---|---|---|---|---|---|---|
| CO | GSAB | PEN | MVN | PUR | ||
| RP-HPLC1 | ||||||
| TL1 | AFerm | 66.79 ± 1.15 a | 22.08 ± 1.49 b | 16.88 ± 2.20 c | 17.86 ± 1.24 c | 3.90 ± 0.74 d |
| ProStab | 2.83 ± 0.57 a | 2.50 ± 0.08 ab | 2.33 ± 0.06 b | 1.88 ± 0.17 c | 0.60 ± 0.15 d | |
| TL2 | AFerm | 17.13 ± 0.43 a | 8.62 ± 0.55 b | 8.77 ± 0.39 b | 9.16 ± 0.49 b | 4.46 ± 0.36 c |
| ProStab | 1.17 ± 0.04 b | 1.11 ± 0.02 b | 1.66 ± 0.25 a | 1.40 ± 0.12 ab | 1.15 ± 0.11 b | |
| TL3 | AFerm | 13.47 ± 0.05 a | 9.61 ± 0.70 c | 11.67 ± 0.71 b | 12.03 ± 0.58 b | 6.83 ± 0.33 d |
| ProStab | 1.56 ± 0.13 b | 1.51 ± 0.03 b | 2.63 ± 0.48 a | 2.30 ± 0.42 a | 2.41 ± 0.07 a | |
| TL4 | AFerm | 48.68 ± 0.58 a | 11.01 ± 0.58 b | 1.54 ± 0.95 c | 1.09 ± 0.17 c | 0.73 ± 0.08 c |
| ProStab | 1.33 ± 0.55 a | 0.94 ± 0.11 a | 0.14 ± 0.09 b | 0.07 ± 0.02 b | 0.03 ± 0.04 b | |
| CHI1 | AFerm | 77.06 ± 9.40 a | 28.64 ± 2.48 b | 22.89 ± 2.40 b | 29.44 ± 2.55 b | 12.78 ± 1.37 c |
| ProStab | 3.88 ± 0.63 | 3.23 ± 0.10 | 3.28 ± 0.16 | 3.38 ± 0.30 | 2.96 ± 0.49 | |
| CHI2 | AFerm | 62.52 ± 12.46 a | 23.05 ± 2.11 b | 15.77 ± 1.93 bc | 20.87 ± 1.73 b | 8.89 ± 0.90 c |
| ProStab | 2.93 ± 0.77 | 2.55 ± 0.06 | 2.31 ± 0.04 | 2.54 ± 0.29 | 1.98 ± 0.42 | |
| SE-HPLC2 | ||||||
| P93 | AFerm | 7.25 ± 0.82 a | 5.80 ± 0.36 b | 5.69 ± 0.82 b | 5.44 ± 0.10 b | 5.07 ± 0.38 b |
| ProStab | 6.35 ± 0.56 | 5.53 ± 0.51 | 5.31 ± 0.55 | 5.14 ± 0.32 | 4.89 ± 0.41 | |
| P67 | AFerm | 6.68 ± 1.07 a | 6.00 ± 0.42 a | 6.54 ± 0.45 a | 6.83 ± 0.56 a | 3.92 ± 0.25 b |
| ProStab | 0.74 ± 0.14 | 0.82 ± 0.06 | 1.22 ± 0.07 | 1.14 ± 0.25 | 1.24 ± 0.51 | |
| PR32 | AFerm | 4.04 ± 0.75 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| ProStab | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PR25 | AFerm | 181.45 ± 43.16 a | 85.81 ± 2.78 b | 70.22 ± 7.48 b | 75.26 ± 1.39 b | 33.09 ± 2.19 c |
| ProStab | 9.67 ± 1.13 | 8.02 ± 1.04 | 9.64 ± 0.73 | 8.94 ± 0.77 | 8.19 ± 0.79 | |
| PR23 | AFerm | 133.92 ± 17.06 a | 52.14 ± 1.53 b | 37.56 ± 3.96 c | 38.55 ± 1.41 c | 15.12 ± 0.77 d |
| ProStab | 6.37 ± 0.82 a | 4.86 ± 0.66 ab | 4.67 ± 0.08 b | 4.21 ± 0.41 b | 2.93 ± 1.02 c | |
| PR22 | AFerm | 63.77 ± 4.93 a | 20.02 ± 1.10 b | 10.86 ± 2.12 c | 10.85 ± 0.89 c | 3.98 ± 0.30 d |
| ProStab | 2.47 ± 0.56 a | 2.17 ± 0.33 a | 1.42 ± 0.16 b | 1.14 ± 0.08 bc | 0.68 ± 0.27 c | |
| PR20 | AFerm | 76.03 ± 7.75 a | 29.36 ± 1.86 b | 21.70 ± 1.87 c | 13.72 ± 0.11 d | 4.89 ± 0.24 e |
| ProStab | 2.91 ± 0.11 a | 2.59 ± 0.17 ab | 2.69 ± 0.28 a | 1.87 ± 0.28 c | 1.99 ± 0.44 bc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, I.; Horvat, I. Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation. Foods 2020, 9, 1534. https://doi.org/10.3390/foods9111534
Lukić I, Horvat I. Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation. Foods. 2020; 9(11):1534. https://doi.org/10.3390/foods9111534
Chicago/Turabian StyleLukić, Igor, and Ivana Horvat. 2020. "Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation" Foods 9, no. 11: 1534. https://doi.org/10.3390/foods9111534
APA StyleLukić, I., & Horvat, I. (2020). Moment of Bentonite Addition, Co-Addition of Tannins, and Bentonite Type Affect the Differential Affinity of Pathogenesis-Related Grape Proteins towards Bentonite during Fermentation. Foods, 9(11), 1534. https://doi.org/10.3390/foods9111534






