Satiety, Taste and the Cephalic Phase: A Crossover Designed Pilot Study into Taste and Glucose Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
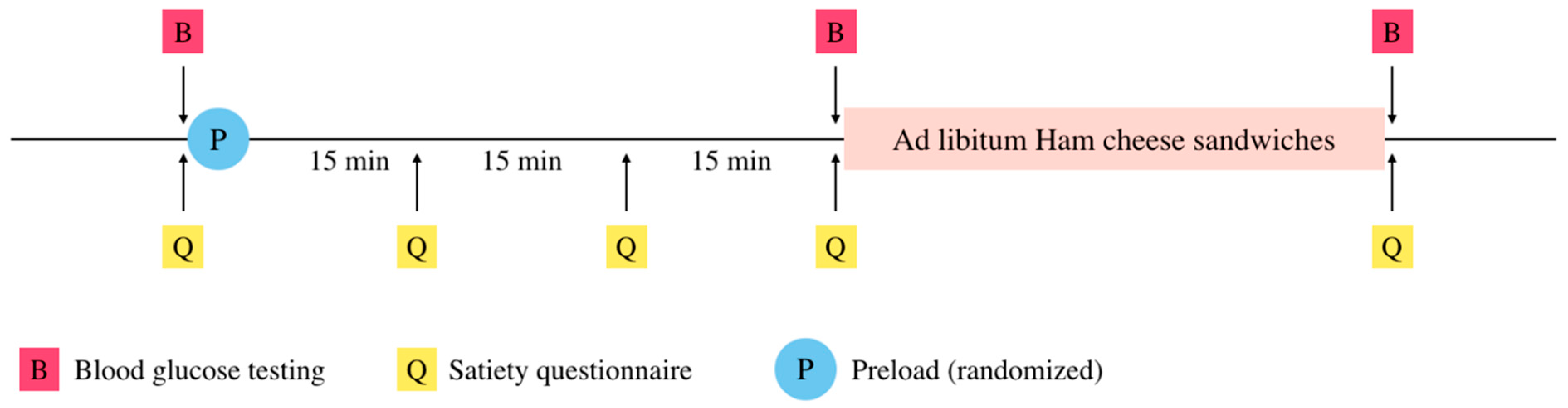
2.2. Study Design
2.3. Pre-Loads
2.4. Test Meal
2.5. Statistics
3. Results
3.1. Maltodextrin Preload Were Perceived No Sweeter than Water, Sucralose as Sweet as Sucrose
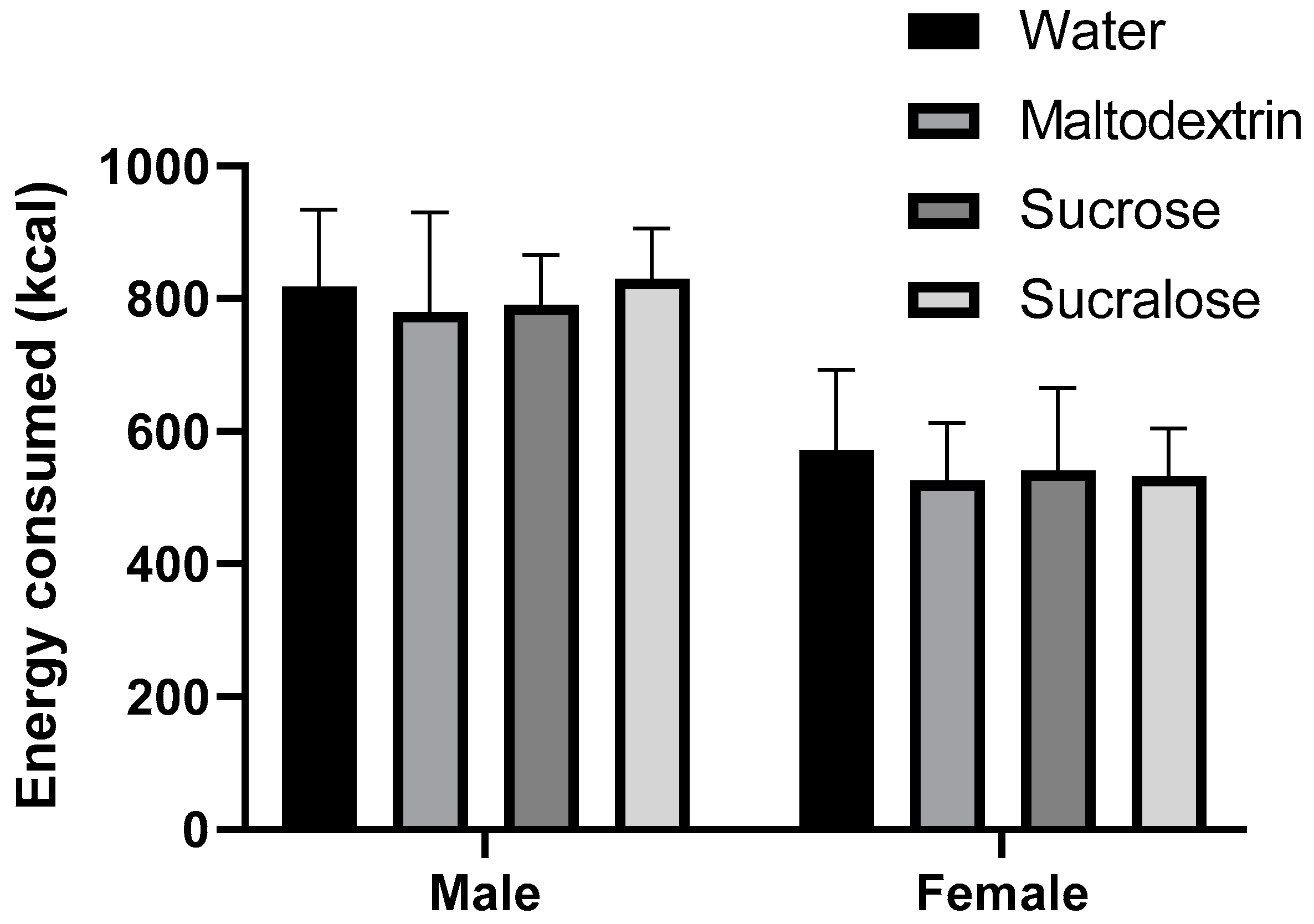
3.2. Consuming Caloric Preload Did Not Reduce Energy Consumption in Test Meal
3.3. Plasma Glucose Concentration Spiked after Tasteless Maltodextrin Preload in Males, but Not after an Equal Amount of Calories from Sucrose
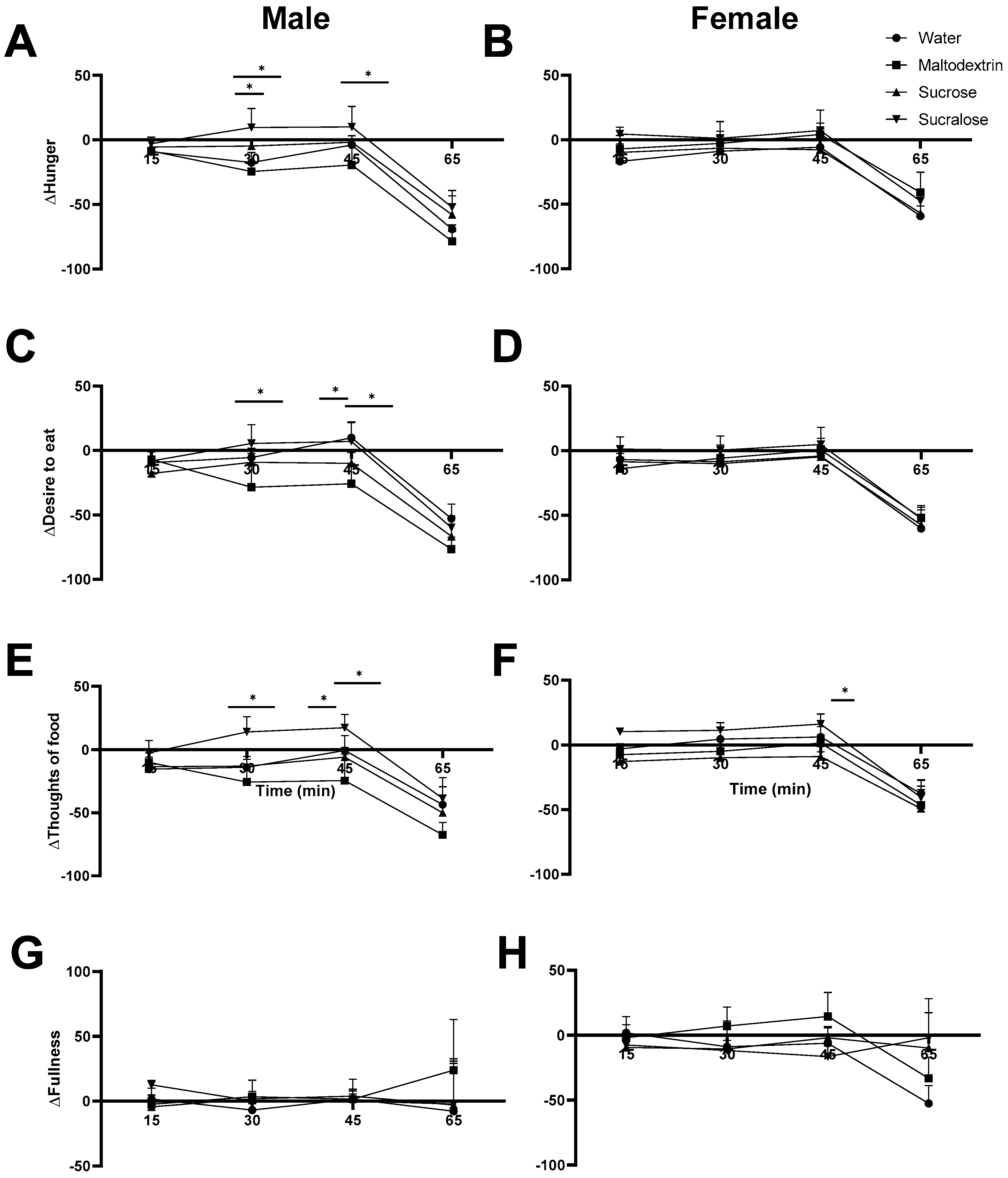
3.4. Satiety Ratings in Males Reflect Trends from Blood Glucose Measurement
4. Discussion
4.1. Plasma Glucose Is Influenced by Cephalic Phase Responses
4.2. Tasteless Versus Sweet-Tasting Preloads
4.3. Energy Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Shearrer, G.E.; O’Reilly, G.A.; Belcher, B.R.; Daniels, M.J.; Goran, M.I.; Spruijt-Metz, D.; Davis, J.N. The impact of sugar sweetened beverage intake on hunger and satiety in minority adolescents. Appetite 2016, 97, 43–48. [Google Scholar] [CrossRef]
- Zijlstra, N.; Mars, M.; De Wijk, R.A.; Westerterp-Plantenga, M.S.; De Graaf, C. The effect of viscosity on ad libitum food intake. Int. J. Obes. 2008, 32, 676–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, B.J.; Castellanos, V.H.; Halford, J.C.; Kilara, A.; Panyam, D.; Pelkman, C.L.; Smith, G.P.; Thorwart, M.L. Volume of food consumed affects satiety in men. Am. J. Clin. Nutr. 1998, 67, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Kendall, F.E.; Marchand, O.; Haszard, J.J.; Venn, B.J. The Comparative Effect on Satiety and Subsequent Energy Intake of Ingesting Sucrose or Isomaltulose Sweetened Trifle: A Randomized Crossover Trial. Nutrients 2018, 10, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, D.S.; Majzoub, J.A.; Al-Zahrani, A.; Dallal, G.E.; Blanco, I.; Roberts, S.B. High glycemic index foods, overeating, and obesity. Pediatrics 1999, 103, E26. [Google Scholar] [CrossRef] [Green Version]
- Kaur, B.; Chin, R.Q.Y.; Camps, S.; Henry, C.J. The impact of a low glycaemic index (GI) diet on simultaneous measurements of blood glucose and fat oxidation: A whole body calorimetric study. J. Clin. Transl. Endocrinol. 2016, 4, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A. Nutrition, Metabolism & Cardiovascular Diseases Glycemic index, glycemic load and glycemic response: An International Scienti fi c Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar]
- Campbell, G.J.; Belobrajdic, D.P.; Bell-anderson, K.S. Determining the Glycaemic Index of Standard and High-Sugar Rodent Diets in C57BL/6 Mice. Nutrients 2018, 10, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glendinning, J.I.; Stano, S.; Holter, M.; Azenkot, T.; Goldman, O.; Margolskee, R.F.; Vasselli, J.R.; Sclafani, A. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R552–R560. [Google Scholar] [CrossRef]
- Güemes, A.; Herrero, P.; Bondia, J.; Georgiou, P. Modeling the effect of the cephalic phase of insulin secretion on glucose metabolism. Med. Biol. Eng. Comput. 2019, 57, 1173–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veedfald, S.; Plamboeck, A.; Deacon, C.F.; Hartmann, B.; Knop, F.K.; Vilsbøll, T.; Holst, J.J. Cephalic phase secretion of insulin and other enteropancreatic hormones in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G43–G51. [Google Scholar] [CrossRef] [Green Version]
- Spector, A.; Glendinning, J. Linking Peripheral Taste Processes to Behavior. Natl. Inst. Health 2009, 19, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Just, T.; Pau, H.W.; Engel, U.; Hummel, T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008, 51, 622–627. [Google Scholar] [CrossRef]
- Teff, K.L.; Engelman, K. Oral sensory stimulation improves glucose tolerance in humans: Effects on insulin, C-peptide, and glucagon. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 270, R1371–R1379. [Google Scholar] [CrossRef]
- Abdallah, L.; Chabert, M.; Louis-Sylvestre, J. Phase Responses to Sweet. Am. J. Clin. Nutr. 1997, 65, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste loss with obesity in mice and men. Int. J. Obes. 2020, 44, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, D.L.; Van Buul, V.J.; Brouns, F.J.P.H. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Babbs, R.K.; Patel, B.; Fobbs, W.; Kroemer, N.B.; Garcia, E.; Yeomans, M.R.; Small, D.M. Integration of sweet taste and metabolism determines carbohydrate reward. Curr. Biol. 2017, 27, 2476–2485.e6. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G. Method in Consumer Research; Woodhead Publishing: Cambridge, UK, 2018; Volume 2, pp. 151–182. [Google Scholar]
- Wiet, G.; Beyts, K. Sensory Characteristics of Sucralose Intensity Sweeteners. J. Food Sci. 1992, 57, 1014–1019. [Google Scholar] [CrossRef]
- Kendig, M.D.; Lin, C.S.; Beilharz, J.E.; Rooney, K.B.; Boakes, R.A. Maltodextrin can produce similar metabolic and cognitive effects to those of sucrose in the rat. Appetite 2014, 77, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G. Measuring Satiation and Satiety. In Methods in Consumer Research, 1st ed.; Ares, G., Varela, P., Eds.; Woodhead Publishing: Cambridge, UK, 2018; Volume 2, ISBN 9780081017432. [Google Scholar]
- Gregersen, N.T.; Flint, A.; Bitz, C.; Blundell, J.E.; Raben, A.; Astrup, A. Reproducibility and power of ad libitum energy intake assessed by repeated single meals. Am. J. Clin. Nutr. 2008, 87, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- Cardello, A.V.; Schutz, H.G. Food appropriateness measures as an adjunct to consumer preference/acceptability evaluation. Food Qual. Prefer. 1996, 7, 239–249. [Google Scholar] [CrossRef]
- Wansink, B.; Painter, J.E.; North, J. Bottomless bowls: Why visual cues of portion size may influence intake. Obes. Res. 2005, 13, 93–100. [Google Scholar] [CrossRef]
- Davy, B.M.; Van Walleghen, E.L.; Orr, J.S. Sex differences in acute energy intake regulation. Appetite 2007, 49, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Shen, W. Gender Diffeences in Insulin Resistance, Body Composition, and Energy Balance. Gend Med. 2009, 6, 60–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cefalu, W.T. Insulin Resistance: Cellular and Clinical Concepts. Exp. Biol. Med. 2001, 226, 13–26. [Google Scholar] [CrossRef]
- Smeets, P.A.; de Graaf, C.; Stafleu, A.; van Osch, M.J.; van der Grond, J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am. J. Clin. Nutr. 2005, 82, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Woodend, D. Consumption of sugars and the regulation of short-term satiety and food intake. Am. J. Clin. Nutr. 2003, 78, 843S–849S. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Lee, J.Y.; Mattes, R.D. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol. Behav. 2017, 181, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Grotz, V.L.; Pi-Sunyer, X.; Porte, D.; Roberts, A.; Richard Trout, J. A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul. Toxicol. Pharm. 2017, 88, 22–33. [Google Scholar] [CrossRef]
- Dokic-Baucal, L.; Dokic, P.; Jakovljevic, J. Influence of different maltodextrins on properties of O/W emulsions. Food Hydrocoll. 2004, 18, 233–239. [Google Scholar] [CrossRef]
- Ciampolini, M.; Bianchi, R. Training to estimate blood glucose and to form associations with initial hunger. Nutr. Metab. 2006, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Juszczak, L.; Gałkowska, D.; Witczak, T.; Fortuna, T. Effect of maltodextrins on the rheological properties of potato starch pastes and gels. Int. J. Food Sci. 2013, 2013, 869362. [Google Scholar] [CrossRef] [Green Version]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1227–G1233. [Google Scholar] [CrossRef]
- Hogenkamp, P.S.; Stafleu, A.; Mars, M.; Brunstrom, J.M.; de Graaf, C. Texture, not flavor, determines expected satiation of dairy products. Appetite 2011, 57, 635–641. [Google Scholar] [CrossRef]
- Camps, G.; Mars, M.; De Graaf, C.; Smeets, P.A. Empty calories and phantom fullness: A randomized trial studying the relative effects of energy density and viscosity on gastric emptying determined by MRI and satiety. Am. J. Clin. Nutr. 2016, 104, 73–80. [Google Scholar] [CrossRef] [Green Version]
- McCrickerd, K.; Chambers, L.; Brunstrom, J.M.; Norton, J.E.; Mills, T.; Yeomans, M.R. Subtle changes in the flavour and texture of a drink enhance expectations of satiety. Appetite 2012, 59, 632. [Google Scholar] [CrossRef]
- Pellegrino, R.; Jones, J.D.; Shupe, G.E.; Luckett, C.R. Sensitivity to viscosity changes and subsequent estimates of satiety across different senses. Appetite 2019, 133, 101–106. [Google Scholar] [CrossRef] [PubMed]
- den Boer, A.; Boesveldt, S.; Lawlor, J.B. How sweetness intensity and thickness of an oral nutritional supplement affects intake and satiety. Food Qual. Prefer. 2019, 71, 406–414. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The Impact of Food Viscosity on Eating Rate, Subjective Appetite, Glycemic Response and Gastric Emptying Rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef] [Green Version]
- Topping, D.L.; Oakenfull, D.; Trimble, R.P.; Illman, R.J. A viscous fibre (methylcellulose) lowers blood glucose and plasma triacylglycerols and increases liver glycogen independently of volatile fatty acid production in the rat. Br. J. Nutr. 1988, 59, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.; Beer, M.U.; Butler, G. Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000, 84, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Lawless, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010; pp. 217–218. [Google Scholar]
- Rolls, B.J.; Kim, S.; Fedoroff, I.C. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol. Behav. 1990, 48, 19–26. [Google Scholar] [CrossRef]
- Black, R.M.; Tanaka, P.; Leiter, L.A.; Anderson, G.H. Soft drinks with aspartame: Effect on subjective hunger, food selection, and food intake of young adult males. Physiol. Behav. 1991, 49, 803–810. [Google Scholar] [CrossRef]
- Black, R.M.; Leiter, L.A.; Anderson, G.H. Consuming aspartame with and without taste: Differential effects on appetite and food intake of young adult males. Physiol. Behav. 1993, 53, 459–466. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sae iab, T.; Dando, R. Satiety, Taste and the Cephalic Phase: A Crossover Designed Pilot Study into Taste and Glucose Response. Foods 2020, 9, 1578. https://doi.org/10.3390/foods9111578
Sae iab T, Dando R. Satiety, Taste and the Cephalic Phase: A Crossover Designed Pilot Study into Taste and Glucose Response. Foods. 2020; 9(11):1578. https://doi.org/10.3390/foods9111578
Chicago/Turabian StyleSae iab, Thanyathorn, and Robin Dando. 2020. "Satiety, Taste and the Cephalic Phase: A Crossover Designed Pilot Study into Taste and Glucose Response" Foods 9, no. 11: 1578. https://doi.org/10.3390/foods9111578
APA StyleSae iab, T., & Dando, R. (2020). Satiety, Taste and the Cephalic Phase: A Crossover Designed Pilot Study into Taste and Glucose Response. Foods, 9(11), 1578. https://doi.org/10.3390/foods9111578





