Effect of Different Combinations of Freezing and Thawing Rates on the Shelf-Life and Oxidative Stability of Ostrich Moon Steaks (M. Femorotibialis medius) under Retail Display Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Overview and Sample Preparation
2.1.1. Experimental Layout
2.1.2. Freezing
2.1.3. Thawing
2.1.4. Shelf-Life
2.2. Physico-Chemical Parameters
2.2.1. Moisture Losses
2.2.2. pH
2.2.3. Surface Colour
CIE L*a*b* and Oxymyoglobin: Metmyoglobin ratio
2.2.4. Lipid Oxidation
2.2.5. Shear Force (Warner–Bratzler)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Moisture Loss
3.2. pH
3.3. Surface Colour
3.4. Lipid Oxidation (2-Thiobarbituric Acid (TBARS))
3.5. Toughness (Shear Force)
3.6. Principle Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barends-Jones, V.; Pienaar, L. The South African Ostrich Industry Footprint; Western Cape Department of Agriculture (WCDoA): Elsenburg, South Africa, 2020. [Google Scholar]
- Department of Agriculture, Forestry & Fisheries (DAFF). A Profile of the South African Ostrich Market Value Chain; Directorate Marketing: Pretoria, South Africa, 2017; pp. 3–18. [Google Scholar]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Meat quality comparison between fresh and frozen/thawed ostrich M. iliofibularis. Meat Sci. 2012, 91, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, Y.H.B. Effects of aging and freezing/thawing sequence on quality attributes of bovine Mm. gluteus medius and biceps femoris. Asian-Australas. J. Anim. Sci. 2017, 30, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Kim, J.H.; Seo, J.K.; Setyabrata, D.; Kim, Y.H.B. Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Sci. 2018, 139, 162–170. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Liesse, C.; Kemp, R.; Balan, P. Evaluation of combined effects of ageing period and freezing rate on quality attributes of beef loins. Meat Sci. 2015, 110, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.B.; Zhou, G.H. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Diaz, M.Y.; Martínez, B.; García-Cachán, M.D. Effect of frozen storage conditions (temperature and length of storage) on microbial and sensory quality of rustic crossbred beef at different stages of aging. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kong, B.; Lui, Q.; Lui, J. Physiochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze/thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.M.; Medel, I.; Beltrán, J.A. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2020, 84, 662–669. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Oxidative stability of previously frozen ostrich Muscularis iliofibularis packaged under different modified atmospheric conditions. Int. J. Food Sci. Technol. 2011, 46, 1171–1178. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Protein and lipid oxidative stability of fresh ostrich M. iliofibularis packaged under different modified atmospheric packaging conditions. Food Chem. 2011, 127, 1659–1667. [Google Scholar] [CrossRef]
- Ambrosiadis, I.; Theodorakakos, N.; Georgakis, S.; Lekas, S. Influence of thawing methods on the quality of frozen meat and drip loss. Fleishwirtschaft 1994, 74, 284–286. [Google Scholar]
- Hong, G.-P.; Park, S.-H.; Kim, J.-Y.; Lee, C.-H.; Lee, S.; Min, S.-G. The effect of thawing rate on the physiochemical properties of frozen ostrich meat. Food Sci. Biotechnol. 2005, 14, 676–680. [Google Scholar]
- Bevilacqua, A.; Zartzky, N.E.; Calvelo, A. Histological measurements of ice in frozen beef. J. Food Technol. 1979, 14, 237–251. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Users Guide, 1st ed.; SAS Institute Inc.: Cary, NC, USA, 2000; Volume 2. [Google Scholar]
- Hofbauer, P.; Smulders, J.M. A summary of methods to assess major physical-chemical and sensory quality traits of fresh (whole tissue) meat. In Game Meat Hygiene in Focus; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; pp. 315–324. [Google Scholar]
- Gonzalez-Sanguinetti, S.; Aňon, M.C.; Calvelo, A. Effect of thawing rate on the exudate production of frozen beef. J. Food Sci. 1985, 50, 697–700. [Google Scholar] [CrossRef]
- Ngapo, T.M.; Babare, I.H.; Reynolds, J.; Mawson, R.F. Freezing and thawing rate effects on drip loss from samples of pork. Meat Sci. 1999, 53, 149–158. [Google Scholar] [CrossRef]
- Wagner, J.R.; Aňon, M.C. Effect of freezing rate on the denaturation of myofibrillar proteins. Food Sci. Technol. 1985, 20, 735–744. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of post mortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Doherty, A.M.; Sheridan, J.J.; Allen, P.; McDowell, D.A.; Blair, I.S. Physical characteristics of lamb primals packaged under vacuum or modified atmospheres. Meat Sci. 1996, 42, 315–324. [Google Scholar] [CrossRef]
- Otremba, M.M.; Dikeman, M.E.; Boyle, E.A.E. Refrigerated shelf-life of vacuum-packaged, previously frozen ostrich meat. Meat Sci. 1999, 52, 279–283. [Google Scholar] [CrossRef]
- Warriss, P.D. Meat hygiene, spoilage and preservation. In Meat Science: An Introductory Text, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; p. 145. [Google Scholar]
- Hoffman, L.C.; Botha, S.S.C.; Britz, T.J. Muscle pH and temperature changes in hot- and cold-deboned ostrich (Struthio camelus var. domesticus) muscularis gastrocnemius, pars interna and muscularis iliofibularis during the first 23 h post mortem. Meat Sci. 2007, 75, 343–349. [Google Scholar] [CrossRef]
- Shange, N.; Gouws, P.A.; Hoffman, L.C. Changes in pH, colour and the microbiology of black wildebeest (Connochaetes gnou) longissimus thoracis et lumborum (LTL) muscle with normal and high (DFD) muscle pH. Meat Sci. 2019, 147, 13–19. [Google Scholar] [CrossRef]
- Neethling, N.E.; Suman, S.P.; Sigge, G.O.; Hoffman, L.C.; Hunt, M.C. Exogenous and endogenous factors influencing color of fresh meat from ungulates. Meat Muscle Biol. 2017, 1, 253–275. [Google Scholar] [CrossRef] [Green Version]
- Lanari, M.C.; Bevilacqua, A.E.; Zaritzky, N.E. Pigment modifications during freezing and frozen storage of packaged beef. J. Food Proc. Eng. 1989, 12, 49–66. [Google Scholar] [CrossRef]
- Farouke, M.M.; Wieliczko, K.J.; Merts, I. Ultra-fast freezing and low storage temperatures are not necessary to maintain the functional properties of manufacturing beef. Meat Sci. 2003, 66, 171–179. [Google Scholar] [CrossRef]
- Farouke, M.M.; Swan, J.E. Effect of muscle condition before freezing and simulated chemical changes during frozen storage on the pH and colour of beef. Meat Sci. 1998, 50, 245–256. [Google Scholar] [CrossRef]
- Tomás, M.C.; Anón, M.C. Study of the influence of freezing rate on lipid oxidation in fish (salmon) and chicken breast muscles. Int. J. Food Sci. Technol. 1990, 25, 718–721. [Google Scholar] [CrossRef]
- Hansen, E.; Juncher, D.; Henckel, P.; Karlsson, A.; Bertelsen, G.; Skibsted, L.H. Oxidative stability of chilled pork chops following long term frozen storage. Meat Sci. 2004, 68, 479–484. [Google Scholar] [CrossRef]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis linne) muscle as influenced by different freeze/thaw cycles. Food Chem. 2001, 72, 207–217. [Google Scholar] [CrossRef]
- Shanks, B.C.; Wulf, D.M.; Maddock, R.J. Technical note: The effect of freezing on Warner-Bratzler shear force values of beef longissimus steaks across several post mortem aging periods. J. Anim. Sci. 2002, 80, 2122–2125. [Google Scholar]
- Petrović, L.; Grujić, R.; Petrović, M. Definition of the optimal freezing rate - 2. Investigation of the physico-chemical properties of beef M. longissimus dorsi frozen at different freezing rates. Meat Sci. 1993, 33, 319–331. [Google Scholar] [CrossRef]
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.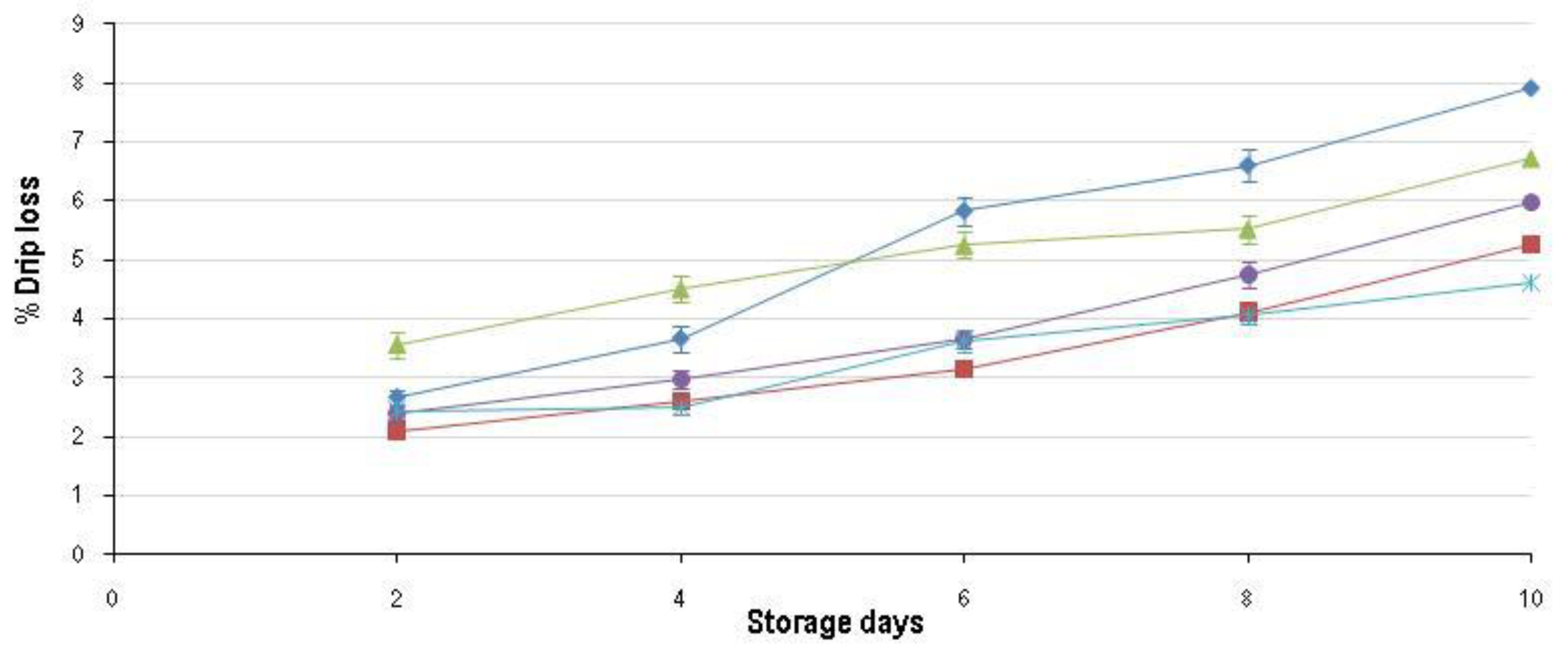
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ) over the 10-day shelf-life trial at ±4 °C.
) over the 10-day shelf-life trial at ±4 °C.
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ).
).
 ); FR_8h (
); FR_8h (  ) and FR_24h (
) and FR_24h (  ).
).
| Treatment | TR_1.5h * | TR_3h | TR_6.5h | TR_14h | TR_21h | Sample Number Per Treatment |
|---|---|---|---|---|---|---|
| FR_1h* | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | nFR_1h = 25 |
| FR_2h | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | nFR_2h = 25 |
| FR_4h | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | nFR_4h = 25 |
| FR_8h | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | nFR_8h = 25 |
| FR_24h | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | n = 5 birds | nFR_24h = 25 |
| Sample number per treatment | nTR_1.5h = 25 | nTR_3h = 25 | nTR_6.5h = 25 | nTR_14h = 25 | nTR_24h = 25 | ntotal = 125 |
| Freeze Treatment | Average Thaw Loss (%) ± SE |
|---|---|
| FR_1h | 2.57 d ± 0.35 |
| FR_2h | 3.00 d ± 0.21 |
| FR_4h | 3.93 c ± 0.35 |
| FR_8h | 5.26 b ± 0.30 |
| FR_24h | 6.24 a ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leygonie, C.; Hoffman, L.C. Effect of Different Combinations of Freezing and Thawing Rates on the Shelf-Life and Oxidative Stability of Ostrich Moon Steaks (M. Femorotibialis medius) under Retail Display Conditions. Foods 2020, 9, 1624. https://doi.org/10.3390/foods9111624
Leygonie C, Hoffman LC. Effect of Different Combinations of Freezing and Thawing Rates on the Shelf-Life and Oxidative Stability of Ostrich Moon Steaks (M. Femorotibialis medius) under Retail Display Conditions. Foods. 2020; 9(11):1624. https://doi.org/10.3390/foods9111624
Chicago/Turabian StyleLeygonie, Coleen, and Louwrens Christiaan Hoffman. 2020. "Effect of Different Combinations of Freezing and Thawing Rates on the Shelf-Life and Oxidative Stability of Ostrich Moon Steaks (M. Femorotibialis medius) under Retail Display Conditions" Foods 9, no. 11: 1624. https://doi.org/10.3390/foods9111624
APA StyleLeygonie, C., & Hoffman, L. C. (2020). Effect of Different Combinations of Freezing and Thawing Rates on the Shelf-Life and Oxidative Stability of Ostrich Moon Steaks (M. Femorotibialis medius) under Retail Display Conditions. Foods, 9(11), 1624. https://doi.org/10.3390/foods9111624





