Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film-Forming Solutions and Chitosan-Based Films
2.2.1. Film-Forming Solutions
2.2.2. Chitosan-Based Films
2.3. Gouda Cheese Preparation
2.4. Experimental Design
2.4.1. Bacterial Inoculation
2.4.2. Fungal Inoculation
2.5. Microbiological Analysis
2.6. Chemical and Physical Analysis
2.6.1. UHPLC
2.6.2. Moisture Content
2.6.3. pH Value
2.6.4. Mechanical Properties
2.6.5. Active Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Chitosan-Based Films
3.1.1. Mechanical Properties
3.1.2. Activity
3.2. Gouda Cheese Spoilage Microbiota Reduction with the CE:CH Film
3.2.1. Bacteria Reduction with Chitosan-Based Films
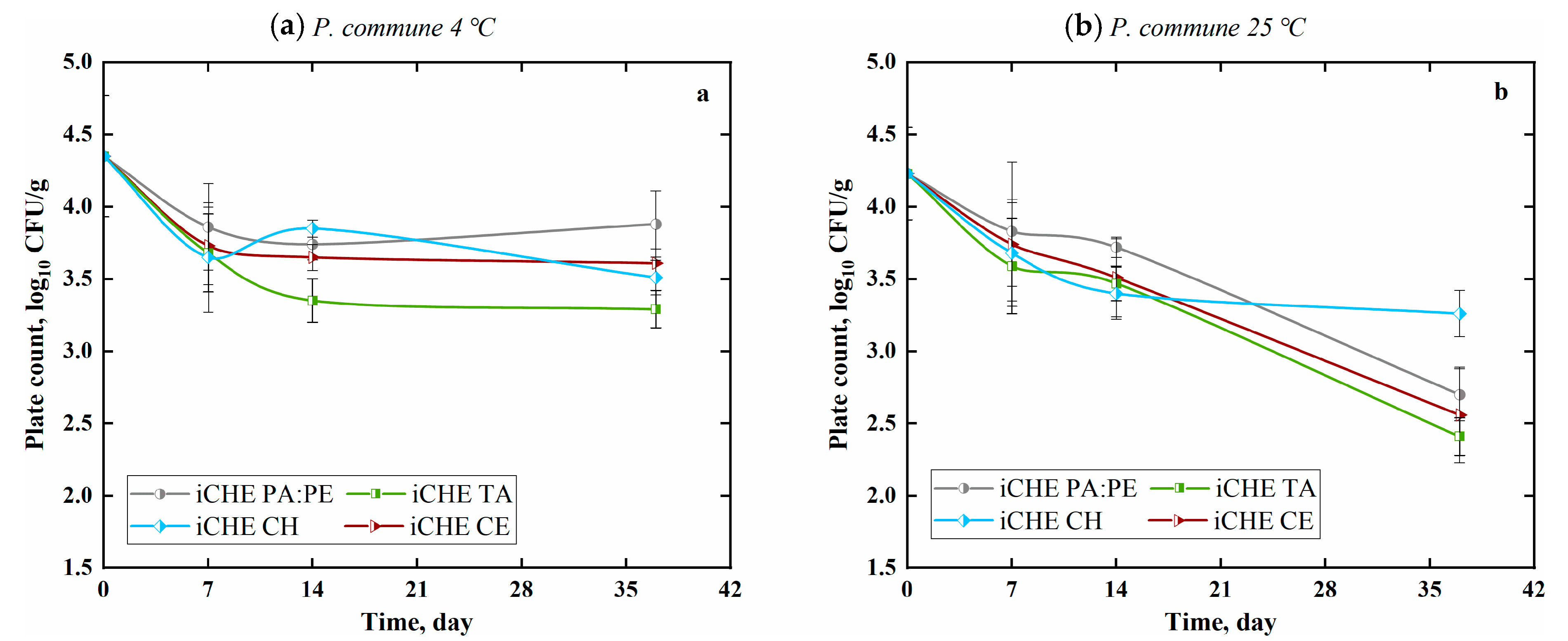
3.2.2. Fungi Reduction with Chitosan-Based Films
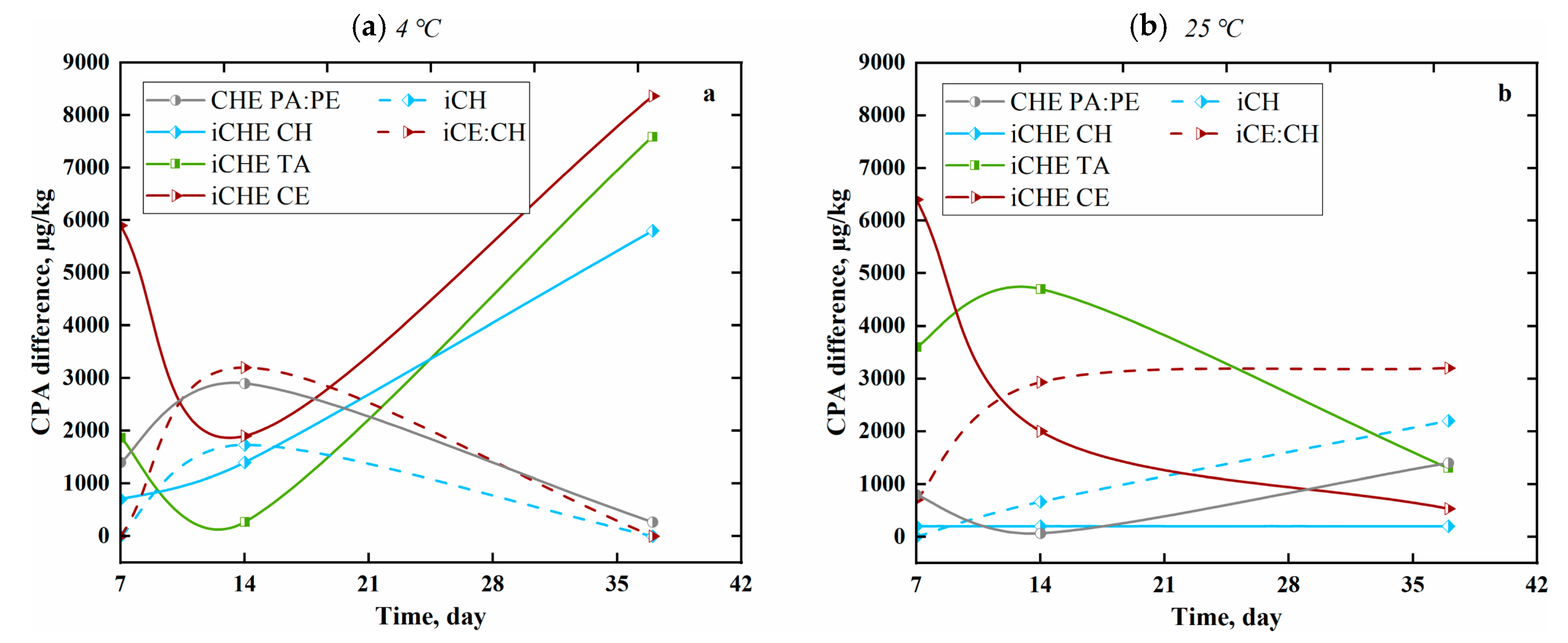
3.2.3. Influence of Chitosan-Based Films on Mycotoxin CPA from Cheese
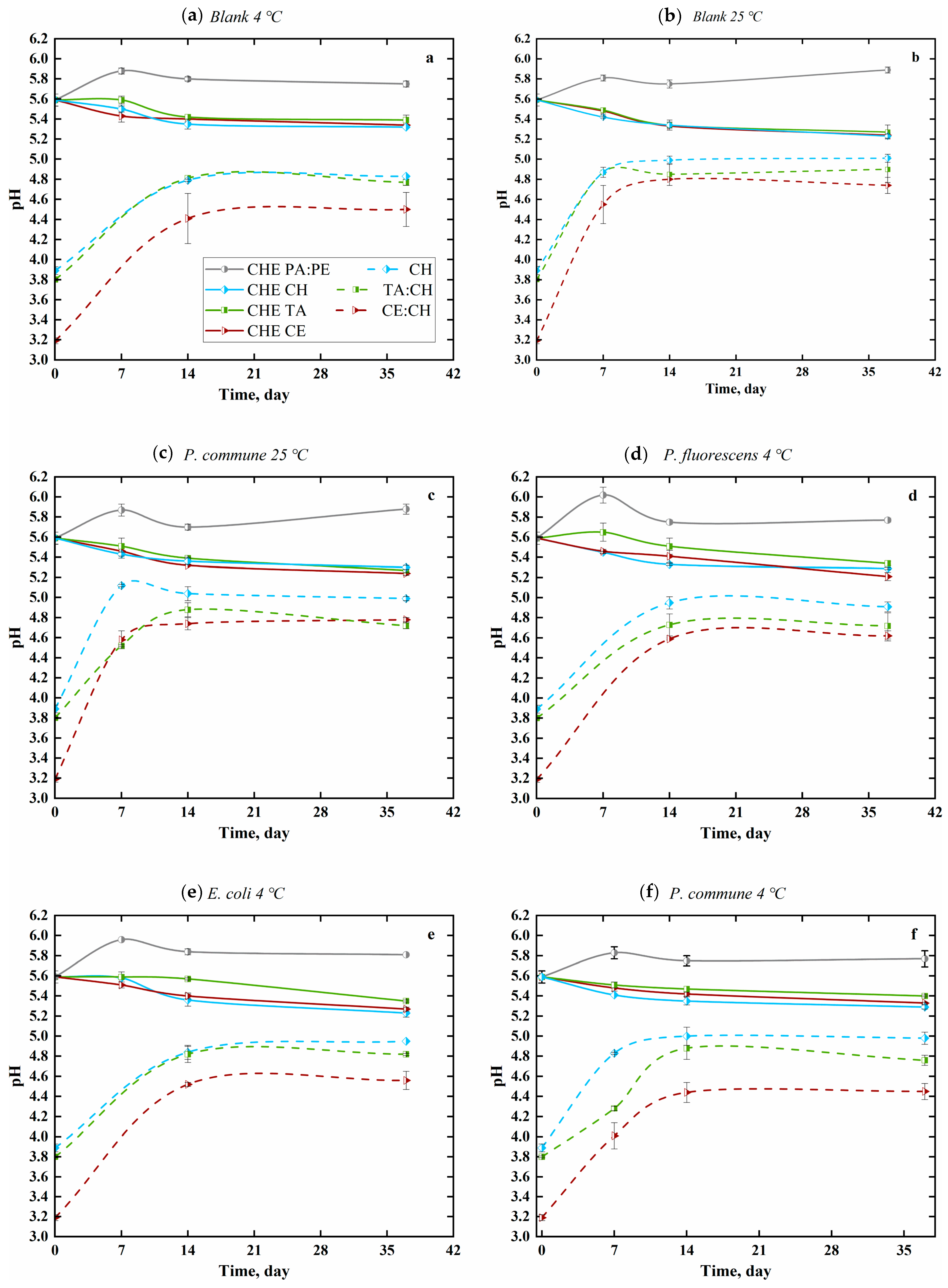
3.3. pH Value
3.4. Moisture Mobility
3.5. Effect of the Film on a Food Safety
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kapoor, R.; Metzger, L.E. Process Cheese: Scientific and Technological Aspects—A Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 194–214. [Google Scholar] [CrossRef]
- Walstra, P.; Noomen, A.; Geurts, T.J. Dutch-Type Varieties. In Cheese: Chemistry, Physics and Microbiology, 2nd ed.; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1993; p. 56. [Google Scholar]
- Lei, T.; Sun, D. Developments of nondestructive techniques for evaluating quality attributes of cheeses: A review. Trends Food Sci. Tech. 2019, 88, 527–542. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Yaman, D.B.; Gonul, S.A. Mycotoxins and mould contamination in cheese: A review. World Mycotoxin J. 2008, 1, 291–298. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Tech. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Saravani, M.; Ehsani, A.; Aliakbarlu, J.; Ghasempour, Z. Gouda cheese spoilage prevention: Biodegradable coating induced by Bunium persicum essential oil and lactoperoxidase system. Food Sci. Nutr. 2018, 7, 959–968. [Google Scholar] [CrossRef]
- Dorner, J.W.; Sobolev, V.S.; Yu, W.; Chu, F.S. Immunochemical Method for Cyclopiazonic Acid. In Mycotoxin Protocols. Methods in Molecular Biology; Trucksess, M.W., Pohland, A.E., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2001; p. 72. [Google Scholar]
- Messini, A.; Buccioni, A.; Minieri, S.; Mannelli, F.; Mugnai, L.; Comparini, C.; Venturi, M.; Viti, C.; Pezzati, A.; Rapaccini, S. Effect of chestnut tannin extract (Castanea sativa Miller) on the proliferation of Cladosporium cladosporioides on sheep cheese rind during the ripening. Int. Dairy J. 2017, 66, 6–12. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Kõrge, K.; Laos, K. The influence of different packaging materials and atmospheric conditions on the properties of pork rinds. J. Appl. Packag. Res. 2019, 11, 1–8. [Google Scholar]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Bajić, M.; Oberlintner, A.; Kõrge, K.; Likozar, B.; Novak, U. Formulation of active food packaging by design: Linking composition of the film-forming solution to properties of the chitosan-based film by response surface methodology (RSM) modelling. Int. J. Biol. Macromol. 2020, 160, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.E.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.A.; Bradic, B.; Novak, U.; Likozar, B. α-Chitin dissolution, N-deacetylation and valorization in deep eutectic solvents. Biopolymers 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wydro, P.; Krajewska, B.; Hac-Wydro, K. Chitosan as a Lipid Binder: A Langmuir Monolayer Study of Chitosan-Lipid Interactions. Biomacromolecules 2007, 8, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol.Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; O′Connell, C.; Viaux, A.; Bou-Maroun, E.; Seuvre, A.; Brachais, C.; Debeaufort, F. Sorption kinetic of aroma compounds by edible bio-based films from marine-by product macromolecules: Effect of relative humidity conditions. Food Chem. 2019, 298, 125064. [Google Scholar] [CrossRef]
- Novak, U.; Bajić, M.; Kõrge, K.; Oberlintner, A.; Murn, J.; Lokar, K.; KTriler, V.; Likozar, B. From waste/residual marine biomass to active biopolymer-based packaging film materials for food industry applications—A review. Phys. Sci. Rev. 2019, 5, 20190099. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Moreira, R. Production of hydrogels with different mechanical properties by starch roasting: A valorization of industrial chestnut by-products. Ind. Crops Prod. 2019, 128, 377–384. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Ferreira, T.; Nascimento-Gonçalves, E.; Seixas, F.; Gil da Costa, R.M.; Martins, T.; Neuparth, M.J.; Pires, M.J.; Lanzarin, G.; Félix, L.; et al. Dietary Supplementation with Chestnut (Castanea sativa) Reduces Abdominal Adiposity in FVB/n Mice: A Preliminary Study. Biomedicines 2020, 8, 75. [Google Scholar] [CrossRef]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible Antimicrobial Films Based on Chitosan Matrix. J. Food Sci. 2002, 67, 1–8. [Google Scholar] [CrossRef]
- Duan, J.; Park, S.I.; Daeschel, M.A.; Zhao, Y. Antimicrobial chitosan-lysozyme (CL) films and coatings for enhancing microbial safety of mozzarella cheese. J. Food Sci. 2007, 72, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bargiacchi, E.; Bellotti, P.; Pinelli, P.; Costa, G.; Miele, S.; Romani, A.; Zambelli, P.; Scardigli, A. Use of Chestnut Tannins Extract as Anti-Oxidant, Anti-Microbial Additve and to Reduce Nitrosamines and Mycotoxins. US Patent Application Publication, 2015. Available online: https://patents.google.com/patent/US20150223512A1/en (accessed on 19 October 2020).
- EUR-lex (European Union Law). Official Journal of the European Union. Available online: https://eur-lex.europa.eu/homepage.html (accessed on 19 October 2020).
- Bajić, M.; Ročnik, T.; Oberlintner, A.; Scognamiglio, F.; Novak, U.; Likozar, B. Natural plant extracts as active components in chitosan-based films: A comparative study. Food Packag. Shelf Life 2019, 21, 100365. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Monaci, L.; Aresta, A. Determination of cyclopiazonic acid in cheese samples using solidphase microextraction and high performance liquid chromatography. Food Chem. 2001, 75, 249–254. [Google Scholar] [CrossRef]
- ASTM International (American Society for Testing and Materials). ASTM Volume 08.01 Plastics (I): C1147-D3159. Designation D 882: Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Available online: https://www.astm.org/Standards/D882 (accessed on 19 October 2020).
- Kõrge, K.; Bajić, M.; Likozar, B.; Novak, U. Active chitosan–chestnut extract films used for packaging and storage of fresh pasta. Int. J. Food Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Bajić, M.; Jalšovec, H.; Travan, A.; Novak, U.; Likozar, B. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef]
- Blaiotta, G.; La Gatta, B.; Di Capua, M.; Di Luccia, A.; Coppola, R.; Aponte, M. Effect of chestnut extract and chestnut fiber on viability of potential probiotic Lactobacillus strains under gastrointestinal tract conditions. Food Microbiol. 2013, 36, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Simard, R.E.; Piette, G.; Bégin, A.; Holley, R.A. Diffusion of Acetic and Propionic Acids from Chitosan-based Antimicrobial Packaging Films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Cheong, E.Y.; Sandhu, A.; Jayabalan, J.; Le, T.T.K.; Nhiep, N.T.; Ho, H.T.M.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Ostry, V.; Toman, J.; Grosse, Y.; Malir, F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018, 11, 135–148. [Google Scholar] [CrossRef]
- Barr, J.G. Effects of Volatile Bacterial Metabolites on the Growth, Sporulation and Mycotoxin Production of Fungi. J. Sci. Food Agric. 1976, 27, 324330. [Google Scholar] [CrossRef]
- Arslan, B.; Soyer, A. Effects of chitosan as a surface fungus inhibitor on microbiological, physicochemical, oxidative and sensory characteristics of dry fermented sausages. Meat Sci. 2018, 145, 107–113. [Google Scholar] [CrossRef]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD–MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D. Morin-Crini, N. Cross-Linked Chitosan-Based Hydrogels for Dye Removal. In Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 36, pp. 381–425. [Google Scholar]
- Ylitalo, R.; Lehtinenc, S.; Wuolijoki, E.; Ylitalo, P.; Lehtimäkid, T. Cholesterol-lowering Properties and Safety of Chitosan. Arzneimittel-Forsch. 2002, 52, 1–7. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Yen, T.-E.; Liu, S.-H.; Chiang, M.-T. Comparative Effects and Mechanisms of Chitosan and Its Derivatives on Hypercholesterolemia in High-Fat Diet-Fed Rats. Int. J. Mol. Sci. 2020, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, N.; Yang, L.; Wang, J.; Song, S.; Nie, D.; Yang, X.; Hou, J.; Wu, A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control 2015, 57, 362–369. [Google Scholar] [CrossRef]
- Thipe, V.C.; Bloebaum, P.; Khoobchandani, M.; Karikachery, A.R.; Katti, K.K.; Katti, K.V. Green nanotechnology: Nanoformulations against toxigenic fungi to limit mycotoxin production. In Nanomycotoxicology; Rai, M., Abd-Elsalam, K.A., Eds.; Elsevier Inc.: Columbia, MO, USA, 2020; pp. 155–188. [Google Scholar]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Effect of hydration on water state, glass transition dynamics and crystalline structure in chitosan films. Carbohydr. Polym. 2019, 206, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Nasri, R.; Hajji, S.; Nigen, M.; Li, S.; Nasri, M. Acetylation degree, a key parameter modulating chitosan rheological, thermal and film-forming properties. Food Hydrocoll. 2019, 87, 48–60. [Google Scholar] [CrossRef]
- Ansari, P.; Häubl, G. Determination of cyclopiazonic acid in white mould cheese by liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) using a novel internal standard. Food Chem. 2016, 211, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int. J. Food Microbiol. 2001, 68, 125–133. [Google Scholar] [CrossRef]
- Purchase, I.F.H. The Acute Toxicity of the Mycotoxin Cyclopiazonic Acid to Rats. Toxicol. Appl. Pharmacol. 1971, 18, 114–123. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess Tech. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Gammariello, D.; Conte, A.; Attanasio, M. A combination of chitosan, coating and modified atmosphere packaging for prolonging Fior di latte cheese shelf life. Carbohydr. Polym. 2009, 78, 151–156. [Google Scholar] [CrossRef]
- Fajardo, P.; Martins, J.T.; Fuciños, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef]
- Ham-Pichavant, F.; Sebe, G.; Pardon, P.; Coma, V. Fat resistance properties of chitosan-based paper packaging for food applications. Carbohydr. Polym. 2005, 61, 259–265. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2019, 126, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
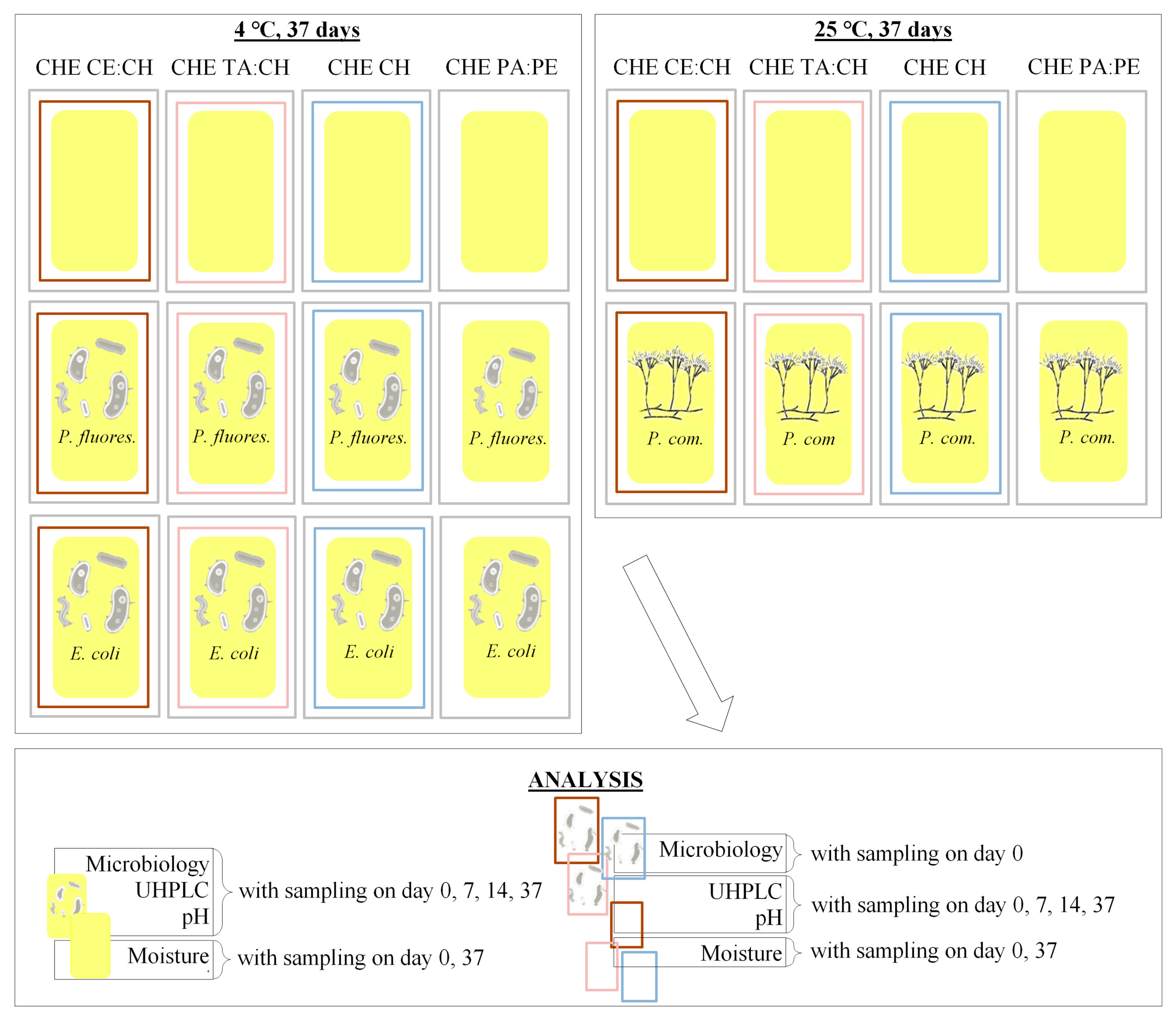

| Name | Abbreviation |
|---|---|
| Inoculated cheese chitosan | iCHE CH |
| Inoculated cheese chestnut extract: chitosan | iCHE CE:CH |
| Inoculated cheese tannic acid: chitosan | iCHE TA:CH |
| Inoculated cheese polyamide: polyethylene | iCHE PA:PE |
| Cheese chitosan | CHE CH |
| Cheese chestnut extract: chitosan | CHE CE:CH |
| Cheese tannic acid: chitosan | CHE TA:CH |
| Cheese polyamide:polyethylene | CHE PA:PE |
| Inoculated chitosan | iCH |
| Inoculated chestnut extract:chitosan | iCE:CH |
| Inoculated tannic acid:chitosan | iTA:CH |
| Chitosan | CH |
| Chestnut extract:chitosan | CE:CH |
| Tannic acid:chitosan | TA:CH |
| Timepoint | Day 0 | Day 37 | |||||
|---|---|---|---|---|---|---|---|
| Sample | Blank 4 °C | Blank 25 °C | P. fluores. 4 °C | E. coli 4 °C | P. com. 4 °C | P. com. 25 °C | |
| Cheese Blank | 33.5 ± 1.8 | 29.5 ± 0.5 | 31.7 ± 1.7 | 27.9 ± 1.0 | 27.9 ± 0.5 | 30.8 ± 1.2 | 29.8 ± 2.0 |
| Cheese CH | 17.8 ± 3.2 | 22.0 ± 1.5 | 19.3 ± 1.6 | 18.0 ± 1.7 | 23.5 ± 1.5 | 19.4 ± 1.5 | |
| Cheese TA | 24.8 ± 2.1 | 26.9 ± 2.1 | 23.1 ± 1.8 | 24.8 ± 1.0 | 26.0 ± 1.4 | 21.9 ± 0.8 | |
| Cheese CE | 26.0 ± 2.4 | 24.2 ± 1.6 | 24.7 ± 1.4 | 23.0 ± 2.4 | 28.4 ± 2.1 | 27.3 ± 1.3 | |
| CH | 4.0 ± 0.4 | 62.6 ± 1.7 | 61.0 ± 1.0 | 65.0 ± 1.0 | 61.6 ± 1.6 | 63.2 ± 3.4 | 61.2 ± 1.2 |
| TA:CH | 2.7 ± 0.4 | 49.8 ± 1.9 | 51.8 ± 1.6 | 52.9 ± 2.3 | 51.9 ± 2.0 | 48.7 ± 3.2 | 51.1 ± 2.1 |
| CE:CH | 3.2 ± 0.2 | 52.0 ± 2.6 | 52.4 ± 3.1 | 49.2 ± 3.1 | 54.2 ± 1.2 | 50.1 ± 3.3 | 55.3 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kõrge, K.; Šeme, H.; Bajić, M.; Likozar, B.; Novak, U. Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods 2020, 9, 1645. https://doi.org/10.3390/foods9111645
Kõrge K, Šeme H, Bajić M, Likozar B, Novak U. Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods. 2020; 9(11):1645. https://doi.org/10.3390/foods9111645
Chicago/Turabian StyleKõrge, Kristi, Helena Šeme, Marijan Bajić, Blaž Likozar, and Uroš Novak. 2020. "Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese" Foods 9, no. 11: 1645. https://doi.org/10.3390/foods9111645
APA StyleKõrge, K., Šeme, H., Bajić, M., Likozar, B., & Novak, U. (2020). Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods, 9(11), 1645. https://doi.org/10.3390/foods9111645







