Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Pomegranates Peels
2.2. Chemicals
2.3. Extraction Methodology
2.4. Methodology of the Determination of TPC
2.5. Determination of Antioxidant Capacity of the PPE (DPPH● Method)
2.6. Box-Behnken Design (BBD) Experiment
2.7. Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
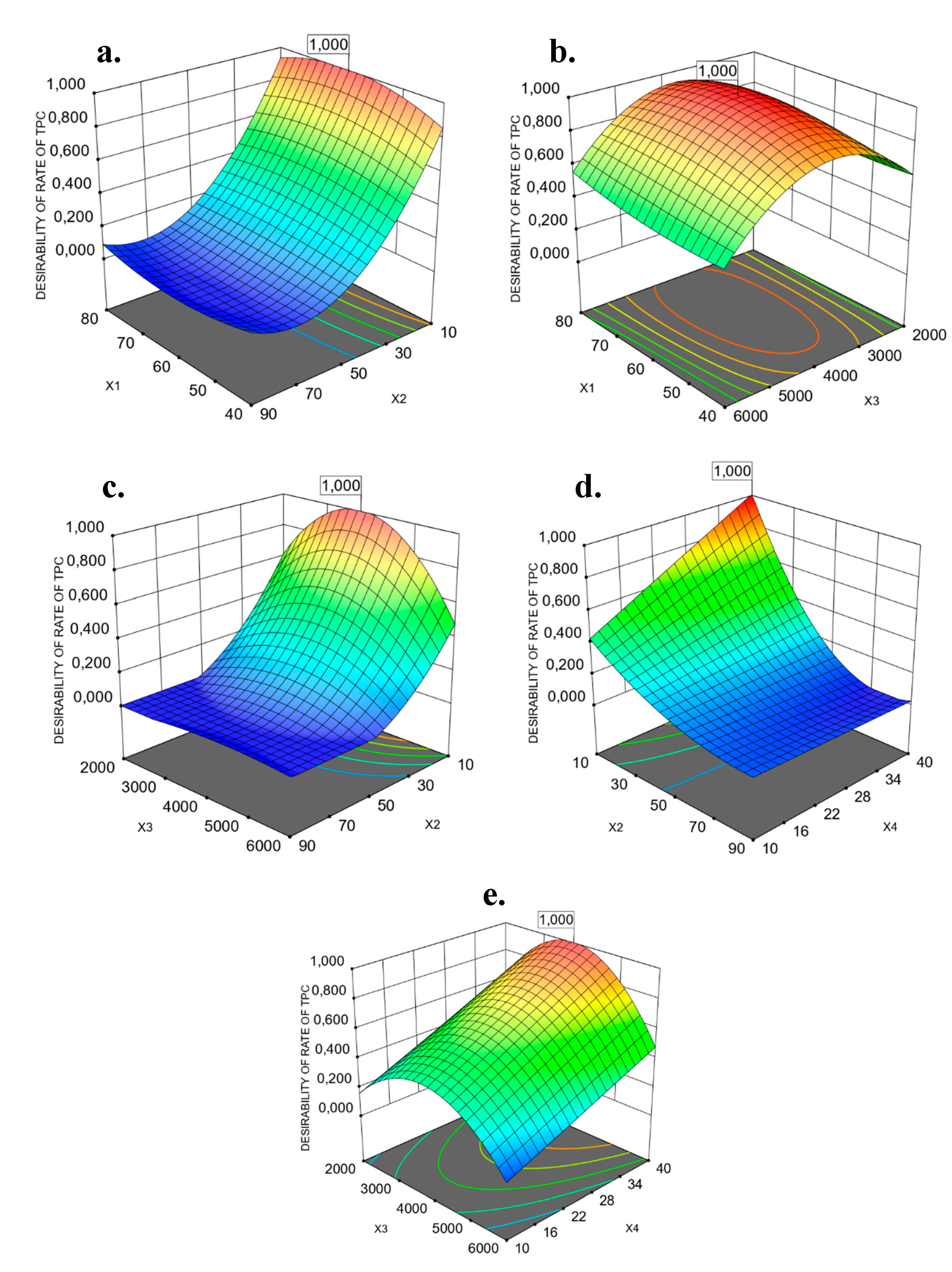
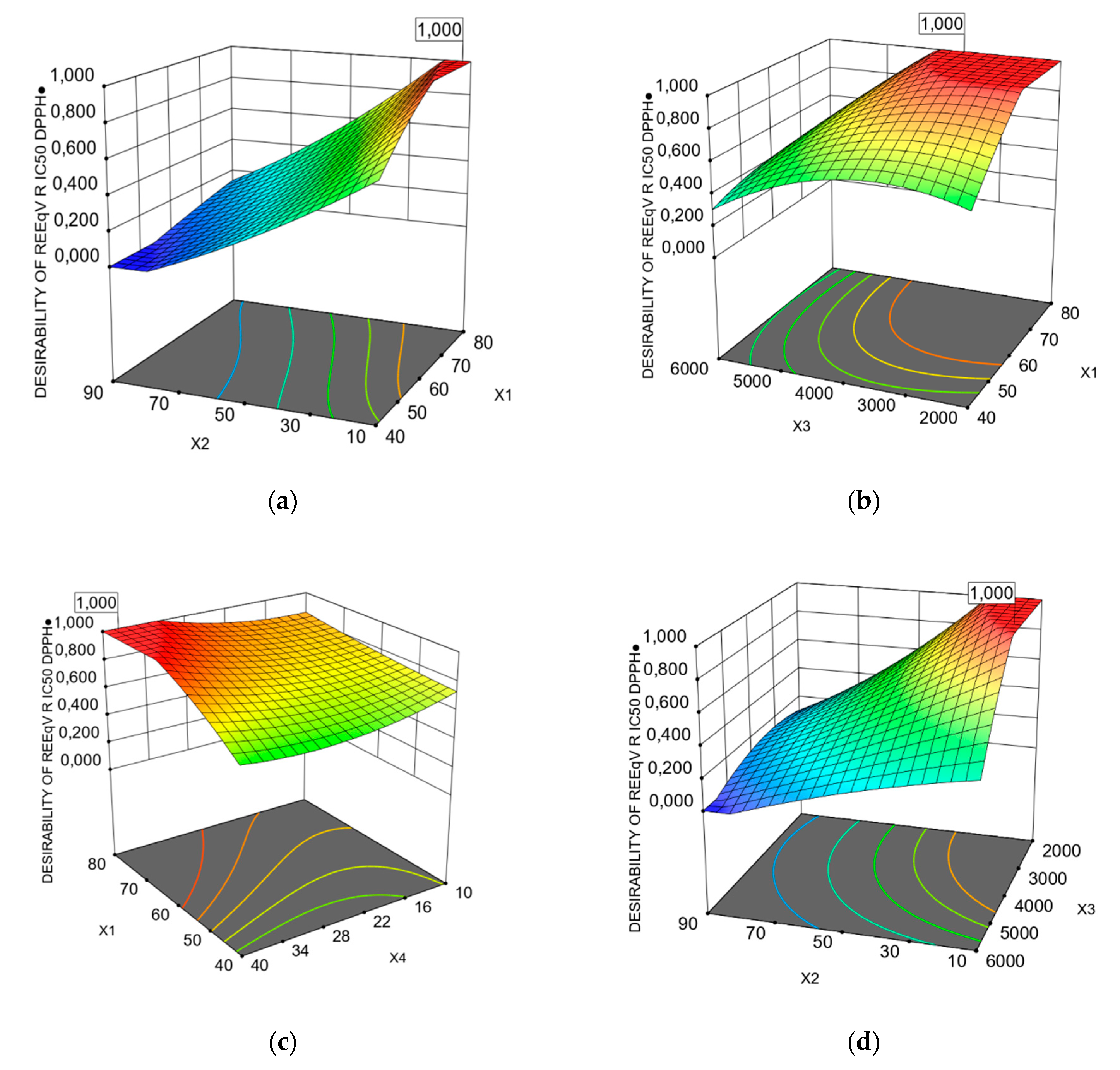
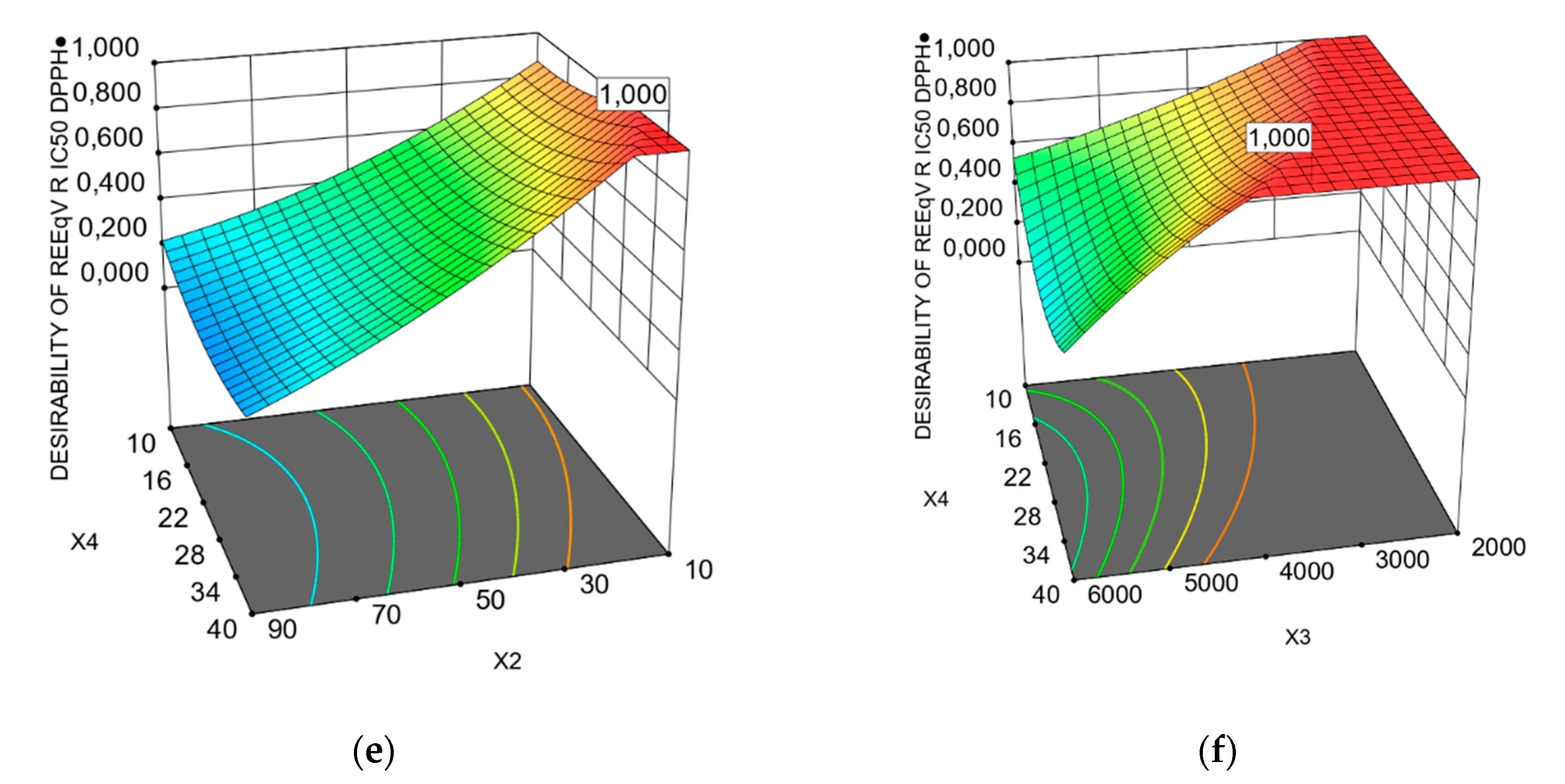
3.1. Predicted Models of Bioactivity Indices by RSM
3.2. Optimization of PP Vacuum Microwave-Assisted Aqueous Extraction
| Independent Variables | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | TPC (mgGAE/g Fresh PP) | EEqVR IC50 DPPH● (L) |
| 77.060 | 10.210 | 3165.020 | 38.590 | 146.442 | |
| 77.050 | 12.480 | 2240.010 | 39.830 | 74.730 | |
X3X4 + 18.73 X12 − 4.81Χ22 − 5.35 X32 − 23.88 X42 + 12.13 X22X4 − 29.49 Χ2Χ42 − 22.30 Χ32Χ4 + 32.24 Χ22Χ42
X1X4 + 11.63 X2X3 − 8.97 X2X4 − 7.25 X3X4 + 8.49 X12 − 7.14 X22 − 0.9221 X32 + 10.11 X42 + 1.32 X12X2− 4.16
X12X3 − 3.24 X1X22 + 4.05 X2X42 − 6.44 X32X4+6.13 X3X42 − 6.74 X12X22
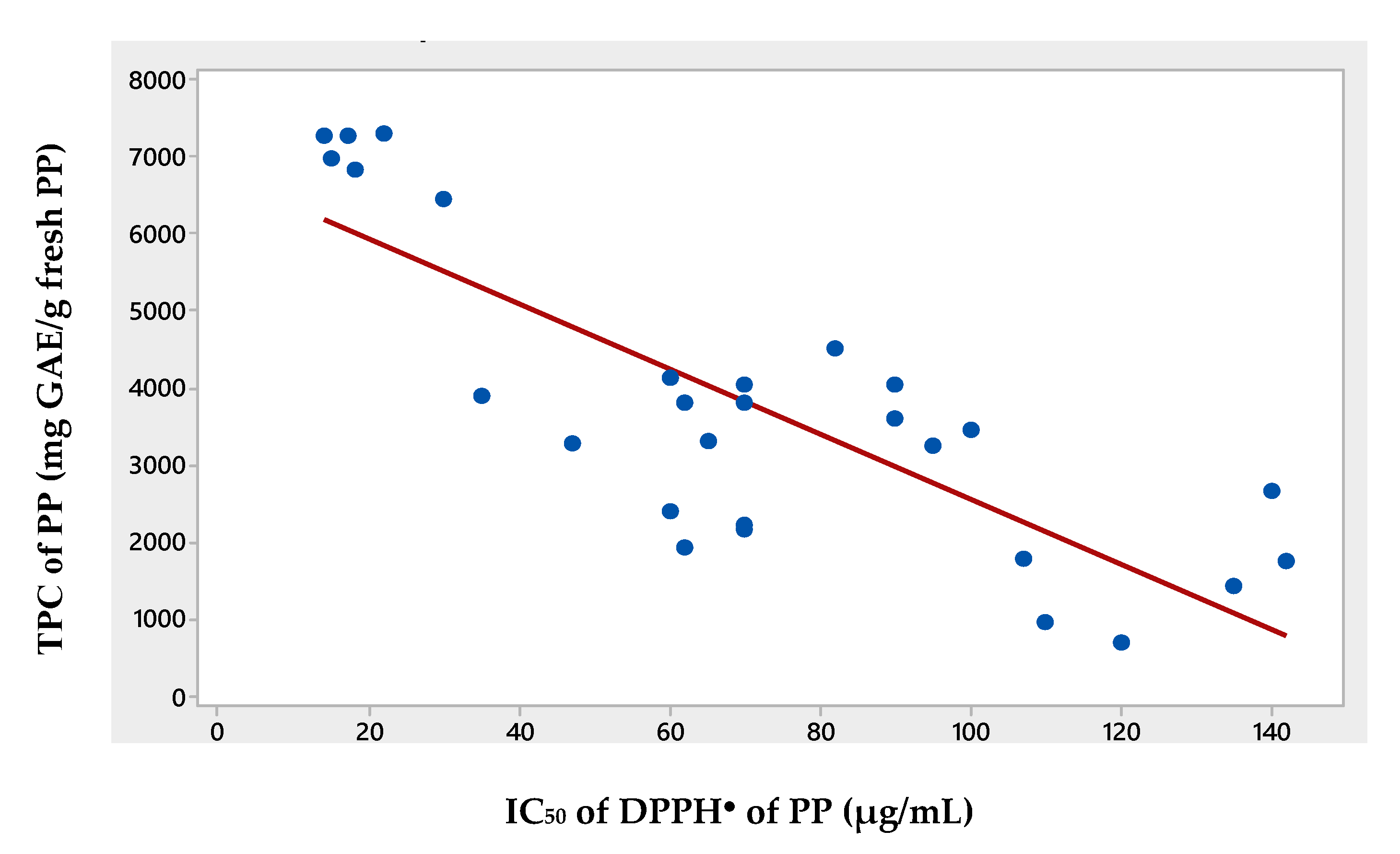
3.3. Correlation of TPC with IC50 of DPPH●
3.4. Modeling of the Extraction of PP Based on the Operational Costs
| Independent Variables | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | RTPC (mgGAE/g Fresh PP)/min | REEqV R IC50 DPPH● (L/min) |
| 61.480 | 10.037 | 3797.240 | 39.924 | 5.542 | |
| 79.158 | 12.127 | 3576.470 | 38.201 | 1.813 | |
0.7728 X2X4 − 0.0365 X3X4 − 0.0994 X12 + 1.13 X22 − 0.3961 X32 + 0.4491 X12X2 + 0.0243 X12X3 − 0.0026 X1X32
− 0.0513 X22X3 + 0.6621 X22X4 + 1.06 X2X32− 0.3431 X32X4 + 0.7116 X12X32 − 0.5249 X22X32
+ 0.1471 X1X4 + 0.2941 X2X3 − 0.1362 X2X4 − 0.1116 X3X4 + 0.1306 X12 + 0.2125 X22 − 0.0138 X32 + 0.1552 X42
+ 0.0601 X12X2 − 0.0753 X12X4 + 0.0157 X1X22 − 0.1170 X22X3 + 0.0685 X2X32 + 0.0000 X2X42 − 0.1786 X32X4
+ 0.1584 X3X42 − 0.1414 X12X22 − 0.0437 X22X32
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Leontopoulos, S. Functional Properties of Goji Berry (Lycium barbarum) Fruit Extracts. In Phytochemicals in Goji Berries; Xingqian Ye, Y.J., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 181–224. ISBN 9780429021749. [Google Scholar]
- Ben-Ali, S.; Akermi, A.; Mabrouk, M.; Ouederni, A. Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 2018, 72, 2087–2100. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, S2675–S2684. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of Goji berry (Lycium barbarum L. and Lycium Chinense mill.) fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and Reactions of Reactive Oxygen Species in Foods. J. Food Sci. 2005, 70, R142–R159. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity Potential of Polyphenolic Compounds in Human Health and their Effectiveness Against Various Food Borne and Plant Pathogens. A Review. J. Food Biosyst. Eng. 2017, 7, 1–19. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Barathikannan, K.; Venkatadri, B.; Khusro, A.; Al-Dhabi, N.A.; Agastian, P.; Arasu, M.V.; Choi, H.S.; Kim, Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Altern. Med. 2016, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- John, K.M.M.; Bhagwat, A.A.; Luthria, D.L. Swarm motility inhibitory and antioxidant activities of pomegranate peel processed under three drying conditions. Food Chem. 2017, 235, 145–153. [Google Scholar] [CrossRef]
- Wang, Z. Extract of Phenolics From Pomegranate Peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, H.S.; Zomorodi, S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. Lebenson. Wiss. Technol. 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef]
- Ho, K.; Ferruzzi, M.G.; Liceaga, A.; San Martin-Gonzalez, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Razzaghi, S.E.; Arabhosseini, A.; Turk, M.; Soubrat, T.; Cendres, A.; Kianmehr, M.H.; Perino, S.; Chemat, F. Operational efficiencies of six microwave based extraction methods for orange peel oil. J. Food Eng. 2019, 241, 26–32. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Wang, J.X.; Wang, G.; Wang, J.Y.; Li, G.K. Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples. J. Chromatogr. A 2009, 1216, 8867–8873. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, B.; Li, L.; Zhu, X. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J. Med. Plants Res. 2011, 5, 1004–1011. [Google Scholar]
- Huang, J.; He, W.; Yan, C.; Du, X.; Shi, X. Microwave assisted extraction of flavonoids from pomegranate peel and its antioxidant activity. BIO Web Conf. 2017, 8, 3008. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Hu, Y. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydr. Polym. 2014, 111, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 2018, 112, 790–802. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken Statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Mansour, E.; Ben Khaled, A.; Lachiheb, B.; Abid, M.; Bachar, K.; Ferchichi, A. Phenolic compounds, antioxidant, and antibacterial activities of peel extract from Tunisian pomegranate. J. Agric. Sci. Technol. 2013, 15, 1393–1403. [Google Scholar]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Wu, P.; Gu, Y.; Zhao, R.; Liu, Y.; Wang, Y.; Lv, G.; Li, Z.; Bao, Y. Residual pomegranate affecting the nonspecific immunity of juvenile Darkbarbel catfish. Fish Shellfish Immunol. 2019, 95, 190–194. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012, 19, 365–372. [Google Scholar] [CrossRef]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Wang, Y.; You, J.; Yu, Y.; Qu, C.; Zhang, H.; Ding, L.; Zhang, H.; Li, X. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008, 110, 161–167. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A. 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Boston, MA, USA, 2013; pp. 15–52. ISBN 978-1-4614-4830-3. [Google Scholar]
- Li, J.; Zu, Y.G.; Fu, Y.J.; Yang, Y.C.; Li, S.M.; Li, Z.N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Khajeh, M.; Akbari Moghaddam, A.R.; Sanchooli, E. Application of Doehlert design in the Optimization of microwave-assisted extraction for determination of zinc and copper in cereal samples using FAAS. Food Anal. Methods 2010, 3, 133–137. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef]
| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Extraction temperature (°C) | X1 | 40 | 60 | 80 |
| Extraction time (min) | X2 | 10 | 50 | 90 |
| Microwave power (W) | X3 | 2000 | 4000 | 6000 |
| Ratio of PP to water (%) | X4 | 10 | 25 | 40 |
| Design Point | Independent Variables in Coded Values | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC (mgGAE/g Fresh PP) | EEqV R IC50 DPPH● (L) | |||||||
| X1 | X2 | X3 | X4 | Measured | Predicted | Measured | Predicted | |
| 1 | −1 | 0 | 1 | 0 | 82.29 ± 0.52 | 74.13 | 53.19 ± 0.17 | 53.44 |
| 2 | 1 | 0 | 0 | 1 | 77.00 ± 0.31 | 77.00 | 64.52 ± 1.81 | 64.54 |
| 3 | 0 | 0 | 0 | 0 | 55.83 ± 0.08 | 67.45 | 35.71 ± 1.46 | 37.45 |
| 4 | 0 | 0 | −1 | 1 | 38.00 ± 0.01 | 38.00 | 36.36 ± 0.44 | 36.36 |
| 5 | 0 | 0 | 0 | 0 | 82.92 ± 0.28 | 67.45 | 38.46 ± 1.12 | 37.45 |
| 6 | −1 | 0 | 0 | 1 | 70.67 ± 0.20 | 70.67 | 37.38 ± 0.22 | 36.86 |
| 7 | 0 | 0 | 0 | 0 | 54.06 ± 0.61 | 67.45 | 35.71 ± 1.46 | 37.45 |
| 8 | 0 | 0 | 1 | 1 | 28.17 ± 0.41 | 28.17 | 33.33 ± 0.11 | 33.33 |
| 9 | 0 | 1 | 0 | 1 | 57.00 ± 0.38 | 60.49 | 29.63 ± 0.22 | 29.88 |
| 10 | −1 | 1 | 0 | 0 | 95.52 ± 0.54 | 89.81 | 35.71 ± 0.46 | 35.71 |
| 11 | 1 | 0 | 1 | 0 | 101.46 ± 0.68 | 82.87 | 27.78 ± 1.00 | 27.48 |
| 12 | 0 | −1 | 0 | −1 | 64.42 ± 0.08 | 60.16 | 33.33 ± 1.07 | 33.03 |
| 13 | −1 | 0 | −1 | 0 | 90.42 ± 0.84 | 85.79 | 27.78 ± 0.09 | 28.03 |
| 14 | 0 | 0 | 0 | 0 | 101.25 ± 0.84 | 67.45 | 35.71 ± 0.25 | 37.45 |
| 15 | 1 | −1 | 0 | 0 | 95.31 ± 0.57 | 130.31 | 35.71 ± 1.11 | 35.71 |
| 16 | 0 | −1 | −1 | 0 | 59.90 ± 0.87 | 60.32 | 41.67 ± 0.01 | 41.69 |
| 17 | 0 | 1 | 0 | −1 | 72.67 ± 0.03 | 80.84 | 58.82 ± 1.35 | 58.52 |
| 18 | 0 | 1 | −1 | 0 | 66.56 ± 0.37 | 67.17 | 17.86 ± 0.37 | 17.88 |
| 19 | 1 | 1 | 0 | 0 | 113.02 ± 0.46 | 105.92 | 30.49 ± 0.01 | 30.49 |
| 20 | −1 | −1 | 0 | 0 | 80.94 ± 0.95 | 104.58 | 26.32 ± 1.46 | 26.32 |
| 21 | 0 | 1 | 1 | 0 | 95.52 ± 0.64 | 119.07 | 40.32 ± 0.66 | 40.35 |
| 22 | 1 | 0 | 0 | −1 | 68.50 ± 0.13 | 68.50 | 55.56 ± 1.12 | 56.13 |
| 23 | 1 | 0 | −1 | 0 | 97.19 ± 0.98 | 99.74 | 71.43 ± 1.41 | 71.13 |
| 24 | 0 | −1 | 0 | 1 | 137.97 ± 0.99 | 108.87 | 40.00 ± 0.60 | 40.25 |
| 25 | 0 | −1 | 1 | 0 | 43.96 ± 0.97 | 45.33 | 17.61 ± 0.59 | 17.63 |
| 26 | 0 | 0 | 0 | 0 | 103.23 ± 0.08 | 67.45 | 41.67 ± 1.95 | 37.45 |
| 27 | 0 | 0 | −1 | −1 | 73.17 ± 0.27 | 73.17 | 45.45 ± 0.21 | 45.45 |
| 28 | −1 | 0 | 0 | −1 | 69.79 ± 0.03 | 69.79 | 66.67 ± 0.05 | 66.69 |
| 29 | 0 | 0 | 1 | −1 | 72.83 ± 0.09 | 72.83 | 71.43 ± 0.27 | 71.43 |
| TPC (mg GAE/g Fresh PP) | EEqV R IC50 DPPH● (L) | ||
|---|---|---|---|
| p Value of the Model | p Value of the Model | ||
| Model | <0.0066 * | Model | <0.0001 * |
| Variables | p Value | Variables | p Value |
| X1 | 0.2296 | X1 | 0.0006 * |
| X2 | 0.0445 * | X2 | 0.7964 |
| X3 | 0.9843 | X3 | 0.7071 |
| X4 | 0.7498 | X4 | 0.0001 * |
| Χ2Χ3 | 0.1428 | X1X2 | 0.0089 * |
| X2X4 | 0.0085 * | X1X3 | <0.0001 * |
| X3X4 | 0.7466 | X1X4 | <0.0001 * |
| X12 | 0.0083 * | X2X3 | <0.0001 * |
| X22 | 0.5051 | X2X4 | <0.0001 * |
| X33 | 0.3903 | X3X4 | 0.0002 * |
| X42 | 0.0047 * | X12 | <0.0001 * |
| X22X4 | 0.2547 | X22 | 0.0002 * |
| X2X42 | 0.0053 * | X32 | 0.3153 |
| X32X4 | 0.0472 * | X42 | <0.0001 * |
| X22X42 | 0.0226 * | X12X2 | 0.3914 |
| X12X3 | 0.0234 * | ||
| X1X22 | 0.0356 * | ||
| X2X42 | 0.0260 * | ||
| X32X4 | 0.0013 * | ||
| X3X42 | 0.0038 * | ||
| X12X22 | 0.0066* | ||
| Lack of fitting | 0.9987 Not significant | 0.9788 Not significant | |
| R2 | 0.8286 | 0.9951 | |
| Adj. R2 | 0.6309 | 0.9805 | |
| RTPC (mg GAE/g Fresh PP)/min | REEqVR IC50 DPPH●(L/min) | ||
|---|---|---|---|
| p Value of the Model | p Value of the Model | ||
| Model | 0.0003 * Significant | Model | <0.0001 * Significant |
| Variables | p Value | Variables | p Value |
| X1 | 0.3535 | X1 | 0.0038 * |
| X2 | <0.0001 * | X2 | <0.0001 * |
| X3 | 0.7962 | X3 | 0.0123 * |
| X4 | 0.8117 | X4 | 0.8830 |
| X1X2 | 0.5063 | X1X2 | 0.0021 * |
| X1X3 | 0.7532 | X1X3 | <0.0001 * |
| X2X3 | 0.1612 | X1X4 | 0.0005 * |
| X2X4 | 0.0011 * | X2X3 | <0.0001 * |
| X3X4 | 0.8093 | X2X4 | 0.0007 * |
| X12 | 0.5063 | X3X4 | 0.0017 * |
| X22 | 0.0001 * | X12 | 0.0008 * |
| X32 | 0.0709 | X22 | 0.0004 * |
| X12X2 | 0.0657 | X32 | 0.4749 |
| X12X3 | 0.9095 | X42 | 0.0003 * |
| X1X32 | 0.9886 | X12X2 | 0.0686 |
| X22X3 | 0.8105 | X12X4 | 0.0337 * |
| X22X4 | 0.0148 * | X22X3 | 0.5170 |
| X2X32 | 0.0013 * | X2X32 | 0.0064 * |
| X32X4 | 0.1399 | X2X42 | 0.0459 * |
| X12X32 | 0.0249 * | X32X4 | - |
| X22X32 | 0.0741 | X3X42 | 0.0010 * |
| X12X22 | 0.0017 * | ||
| X22X32 | 0.0120 * | ||
| Lack of fitting | 0.9228 Not significant | 0.7813 Not significant | |
| R2 | 0.9823 | 0.9985 | |
| Adj. R2 | 0.9293 | 0.9915 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity. Foods 2020, 9, 1655. https://doi.org/10.3390/foods9111655
Skenderidis P, Leontopoulos S, Petrotos K, Giavasis I. Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity. Foods. 2020; 9(11):1655. https://doi.org/10.3390/foods9111655
Chicago/Turabian StyleSkenderidis, Prodromos, Stefanos Leontopoulos, Konstantinos Petrotos, and Ioannis Giavasis. 2020. "Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity" Foods 9, no. 11: 1655. https://doi.org/10.3390/foods9111655
APA StyleSkenderidis, P., Leontopoulos, S., Petrotos, K., & Giavasis, I. (2020). Optimization of Vacuum Microwave-Assisted Extraction of Pomegranate Fruits Peels by the Evaluation of Extracts’ Phenolic Content and Antioxidant Activity. Foods, 9(11), 1655. https://doi.org/10.3390/foods9111655








