Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Legume Protein Isolate Extraction
2.3. Protein Hydrolysis
2.3.1. Simulated Gastrointestinal Digestion
2.3.2. Alcalase Enzymatic Digestion
2.4. Degree of Hydrolysis (DH)
2.5. Gel Electrophoresis Analysis SDS–PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis)
2.6. Identification and Characterization of Potentially Bioactive Peptides
2.7. Antioxidant Capacity Assays
2.7.1. ABTS Radical Scavenging Activity
2.7.2. DPPH Radical Scavenging Activity
2.7.3. Nitric Oxide (NO) Radical Scavenging
2.8. Biochemical Analyses to Determine Anti-Adipogenic Potential
2.8.1. Lipase Activity Measurement Using pH Indicator-Based Lipase Assay
2.8.2. Hydroxy-3-methylglutaryl Coenzyme a Reductase (HMG-CoA Reductase) Activity Assay
2.9. Isobolographic Analysis of PL Inhibitory Activity by LPH
2.10. Molecular Docking (In Silico Analysis)
2.11. Statistical Analysis
3. Results
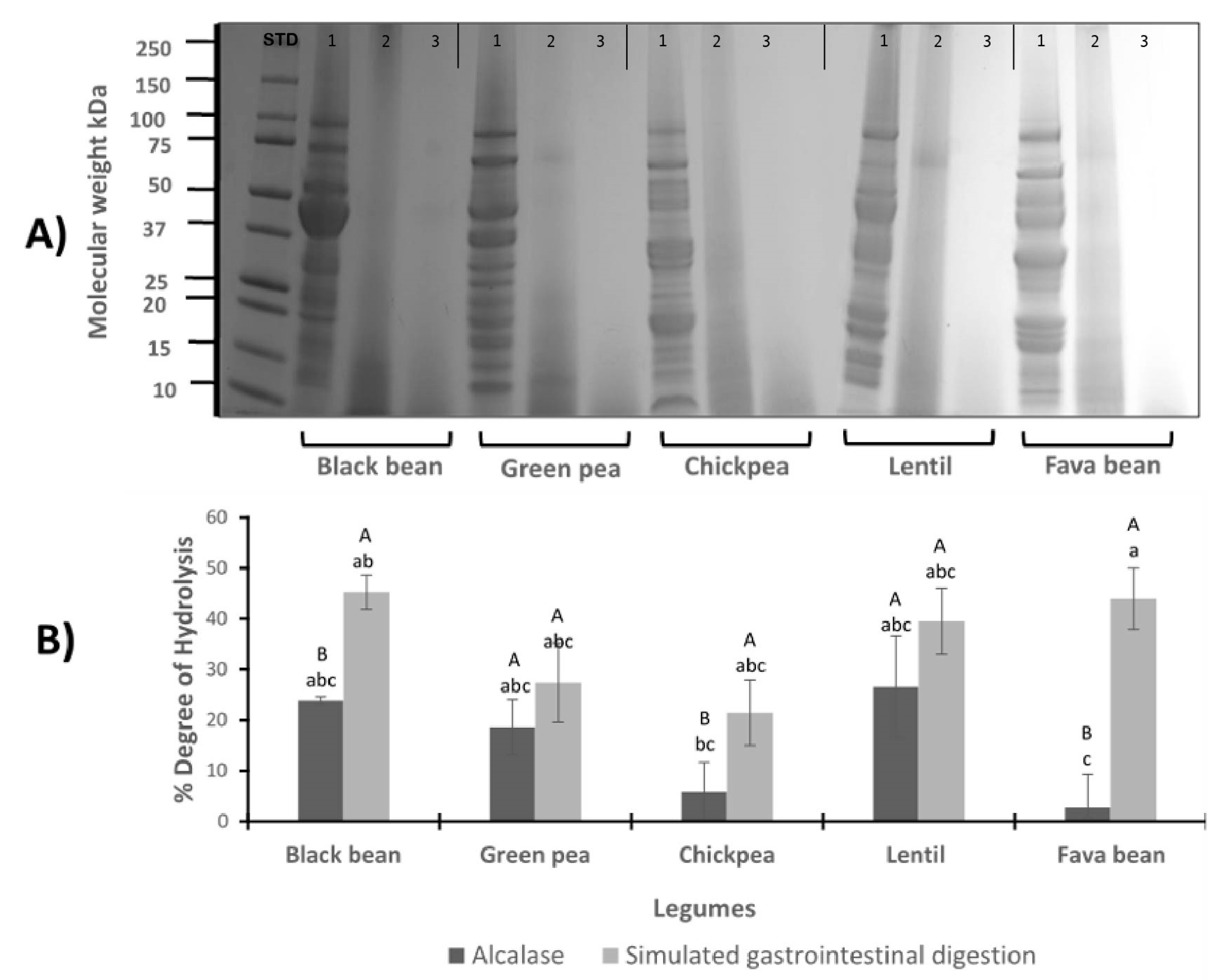
3.1. Protein Profile for LPI and LPH by Gel Electrophoresis Analysis
3.2. Degree of Hydrolysis
3.3. Peptide Sequences and Predicted Bioactivity
3.4. Antioxidant Capacity
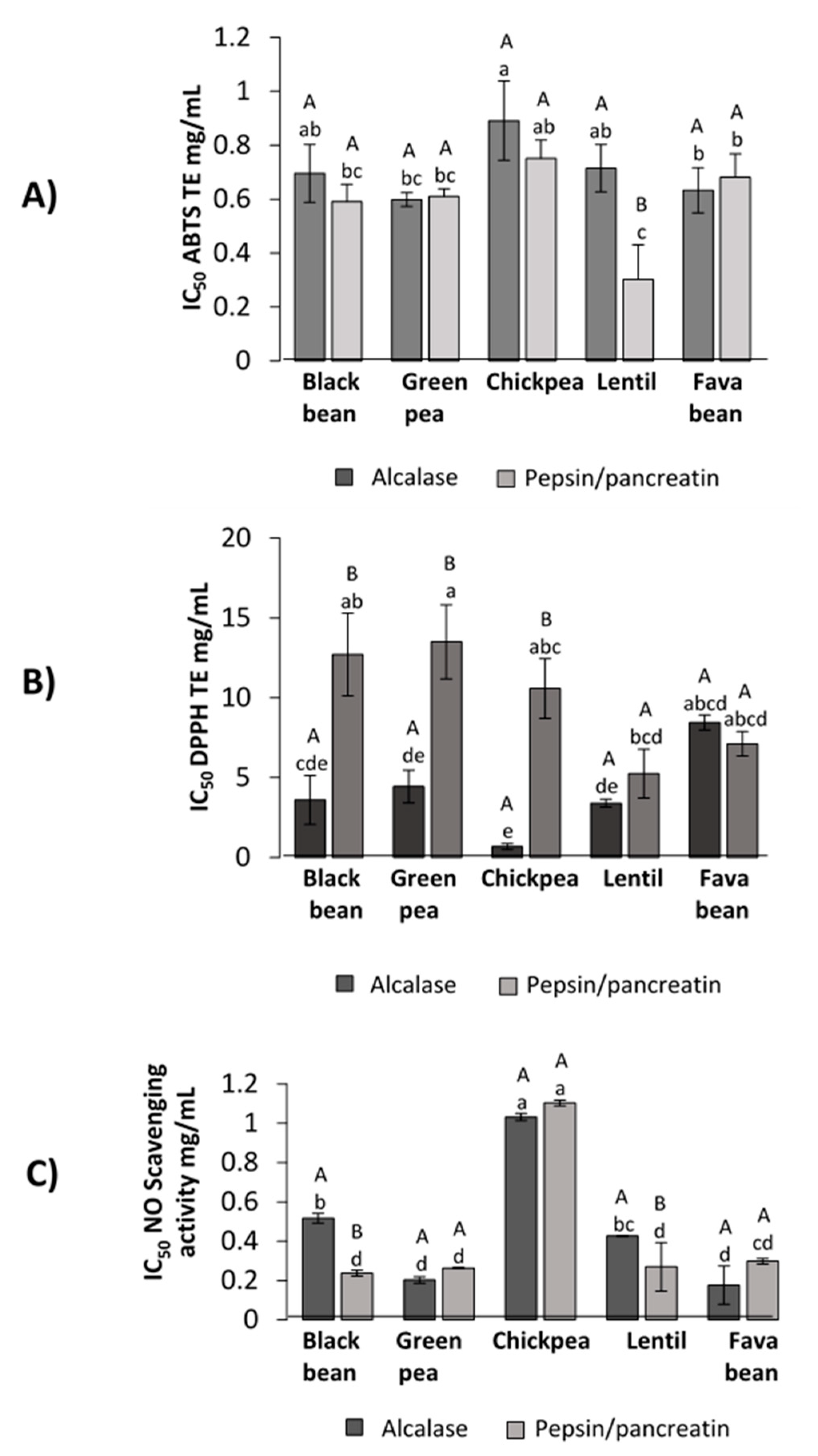
3.4.1. ABTS Radical Scavenging
3.4.2. DPPH Inhibition Capacity
3.4.3. Nitric Oxide (NO) Scavenging Capacity
3.5. Anti-Adipogenic Potential
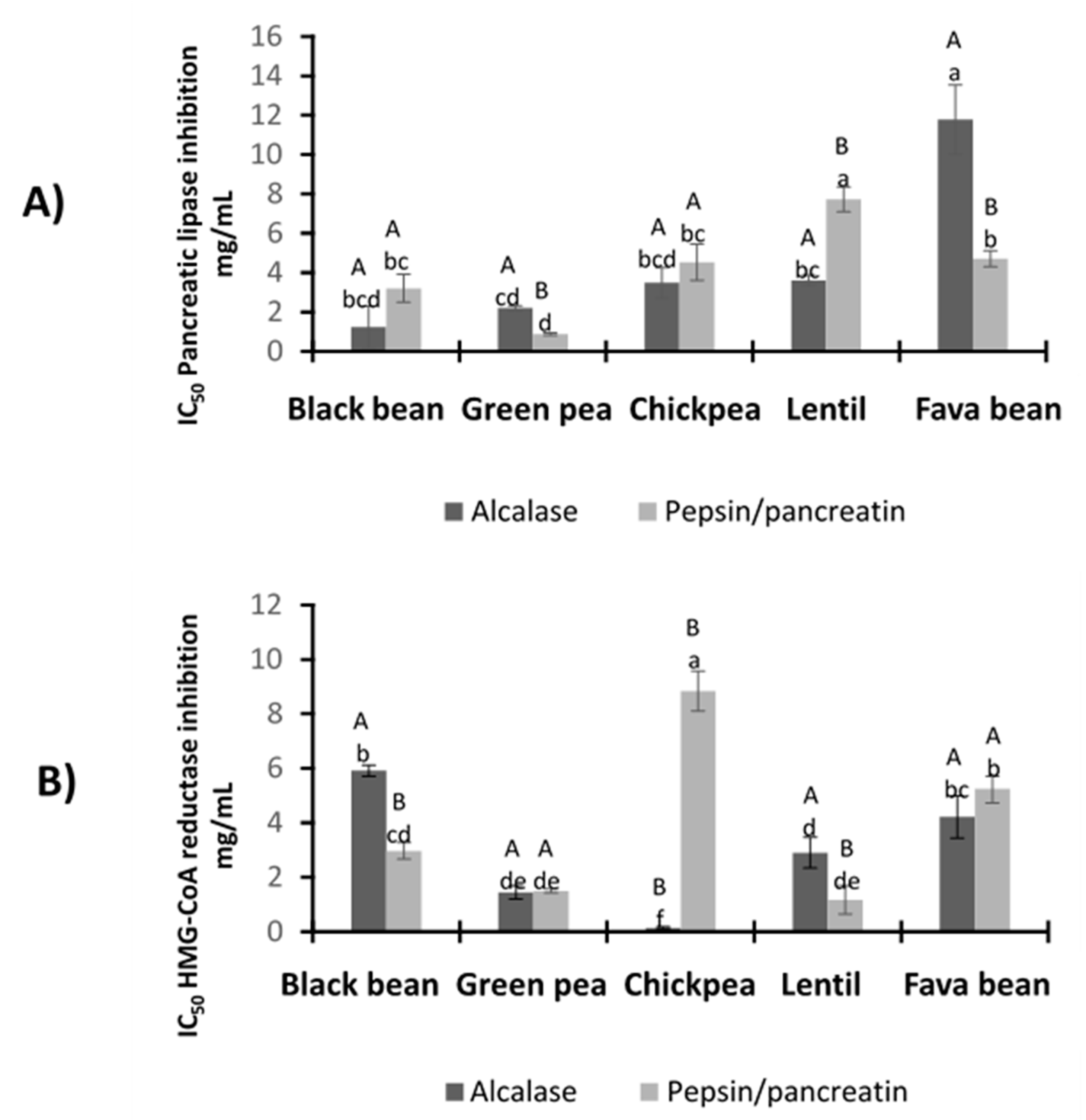
3.5.1. Pancreatic Lipase Inhibitory Activity
3.5.2. HMG-CoA Reductase Inhibitory Activity
3.5.3. Isobolograms of the LPH Interactions
3.6. Molecular Docking Study of Peptides Inhibiting Pancreatic Lipase and HMG-CoA Reductase
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Feingold, K.R.; Grunfeld, C. Obesity and Dyslipidemia; MDText.com, Inc.: South Darmouth, MA, USA, 2000. [Google Scholar]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 April 2019).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Waalen, J. The genetics of human obesity. Transl. Res. 2014, 164, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Savage, P.D. Obesity in coronary heart disease: An unaddressed behavioral risk factor. Prev. Med. 2017, 104, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannenberg, A.J.; Berger, N.A. Obesity, Inflammation and Cancer; Dannenberg, A.J., Berger, N.A., Eds.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-6818-9. [Google Scholar]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and Type 2 diabetes: What can be unified and what needs to be individualized? Diabetes Care 2011, 34, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-approved anti-obesity drugs in the United States. Am. J. Med. 2016, 129, 879.e1–879.e6. [Google Scholar] [CrossRef]
- Leung, W.Y.S.; Neil Thomas, G.; Chan, J.C.N.; Tomlinson, B. Weight management and current options in pharmacotherapy: Orlistat and sibutramine. Clin. Ther. 2003, 25, 58–80. [Google Scholar] [CrossRef]
- Gupta, R. Traumatic brain injury. In Rapid Review Anesthesiology Oral Boards; Gupta, R., Tran, M.C.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 73–78. ISBN 9780128040645. [Google Scholar]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin adverse effects. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Olias, R. Beneficial effects of legumes in gut health. Curr. Opin. Food Sci. 2017, 14, 32–36. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C. Plant Proteins from Legumes; Springer: Cham, Switzerland, 2019; pp. 223–265. ISBN 978-3-319-78029-0. [Google Scholar]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Santhakumar, A.B.; Luo, J.; Blanchard, C.L. Therapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J. Appl. Biomed. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and isoflavones in gastrointestinal digests contribute to the anti-inflammatory potential of cooked or germinated desi and kabuli chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Mojica, L.; de Mejía, E.G.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Luna-Vital, D.; de Mejía, E.G. Peptides from legumes with antigastrointestinal cancer potential: Current evidence for their molecular mechanisms. Curr. Opin. Food Sci. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Mojica, L.; Chen, K.; de Mejía, E.G. Impact of commercial precooking of common bean (Phaseolus vulgaris) on the generation of peptides, after pepsin-pancreatin hydrolysis, capable to inhibit Dipeptidyl Peptidase-IV. J. Food Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; de Mejia, E.G.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef]
- de los Angeles Camacho-Ruiz, M.; Mateos-Díaz, J.C.; Carrière, F.; Rodriguez, J.A. A broad pH range indicator-based spectrophotometric assay for true lipases using tributyrin and tricaprylin. J. Lipid Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Mojica, L.; de Mejia, E.G.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform 2009, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T. A merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J. Comput. Chem. 1996, 17, 520–552. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; de Mejía, E.G. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Stanojevic, S.; Pesic, M.; Pavlicevic, M.; Zlatkovic, B.; Jankovic, M. Functional properties of protein hydrolysates from pea (Pisum sativum, L) seeds. Int. J. Food Sci. Technol. 2012. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-converting enzyme inhibitory properties of lentil protein hydrolysates: Determination of the kinetics of inhibition. Food Chem. 2011. [Google Scholar] [CrossRef]
- Rasmussen, T.P. Genomic medicine and lipid metabolism. In Translational Cardiometabolic Genomic Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–118. ISBN 9780127999616. [Google Scholar]
- Hakes, L.; Lovell, S.C.; Oliver, S.G.; Robertson, D.L. Specificity in protein interactions and its relationship with sequence diversity and coevolution. Proc. Natl. Acad. Sci. USA 2007, 104, 7999–8004. [Google Scholar] [CrossRef] [Green Version]
- Gane, P.J.; Dean, P.M. Recent advances in structure-based rational drug design. Curr. Opin. Struct. Biol. 2000, 10, 401–404. [Google Scholar] [CrossRef]
- Man, D. Enzymic hydrolysis of food proteins. J. Food Eng. 1989, 9, 165–166. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. One pot use of combilipases for full modification of oils and fats: Multifunctional and heterogeneous substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Jarpa-Parra, M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Ahmadifard, N.; Murueta, J.H.C.; Abedian-Kenari, A.; Motamedzadegan, A.; Jamali, H. Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with SDS-PAGE. J. Food Sci. Technol. 2016, 53, 1279–1284. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of physicochemical properties of 7S and 11S globulins from pea, fava bean, cowpea, and french bean with those of soybean french bean 7S globulin exhibits excellent properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef]
- Rangel, A.; Domont, G.B.; Pedrosa, C.; Ferreira, S.T. Functional properties of purified vicilins from cowpea (Vigna unguiculata) and pea (Pisum sativum) and cowpea protein isolate. J. Agric. Food Chem. 2003, 51, 5792–5797. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Sila, A.; Przybylski, R.; Nedjar-Arroume, N.; Makhlouf, I.; Blecker, C.; Attia, H.; Dhulster, P.; Bougatef, A.; Besbes, S. Purification and identification of novel antioxidant peptides from enzymatic hydrolysate of chickpea (Cicer arietinum L.) protein concentrate. J. Funct. Foods 2015, 12, 516–525. [Google Scholar] [CrossRef]
- Liu, C.; Bhattarai, M.; Mikkonen, K.S.; Heinonen, M. Effects of enzymatic hydrolysis of fava bean protein isolate by alcalase on the physical and oxidative stability of oil-in-water emulsions. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Lee, S.S.; Mohd Esa, N.; Loh, S.P. In vitro inhibitory activity of selected legumes against pancreatic lipase. J. Food Biochem. 2015, 39, 485–490. [Google Scholar] [CrossRef]
- Soares, R.; Mendonça, S.; de Castro, L.Í.Í.; Menezes, A.; Arêas, J. Major peptides from Amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two peptides from Soy β-conglycinin induce a hypocholesterolemic effect in HepG2 cells by a statin-Like mechanism: Comparative in vitro and in silico modeling studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Zanoni, C.; Arnoldi, A. IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. J. Funct. Foods 2015, 14, 469–478. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef]
- Oseguera Toledo, M.E.; de Mejia, E.G.; Sivaguru, M.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) protein-derived peptides increased insulin secretion, inhibited lipid accumulation, increased glucose uptake and reduced the phosphatase and tensin homologue activation in vitro. J. Funct. Foods 2016, 27, 160–177. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Lima, S.L.S.; Alves, N.E.G.; Assis, A.; Moreira, M.E.C.; Toledo, R.C.L.; Rosa, C.O.B.; Teixeira, O.R.; Bassinello, P.Z.; De Mejía, E.G.; et al. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.A.; De Mejía, E.G.; Mendoza, S.; Loarca-Piña, G. Peptides present in the non-digestible fraction of common beans (Phaseolus vulgaris L.) inhibit the angiotensin-I converting enzyme by interacting with its catalytic cavity independent of their antioxidant capacity. Food Funct. 2015, 6, 1470–1479. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Synergism between resveratrol and other phytochemicals: Implications for obesity and osteoporosis. Mol. Nutr. Food Res. 2011, 55, 1177–1185. [Google Scholar] [CrossRef]
- Leena, M.M.; Silvia, G.; Kannadasan, V.; Jeyan, M.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- do Evangelho, J.A.; Vanier, N.L.; Pinto, V.Z.; De Berrios, J.J.; Dias, A.R.G.; da Zavareze, E.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef]
- Kou, X.; Gao, J.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Habu, J.B.; Ibeh, B.O. In vitro antioxidant capacity and free radical scavenging evaluation of active metabolite constituents of Newbouldia laevis ethanolic leaf extract. Biol. Res. 2015, 48, 16. [Google Scholar] [CrossRef] [Green Version]



| Sample | Molecular Mass (Da) | Peptide | Bioactive Sequence | pI | Net Charge | Hydrophobicity (kcal/mol) | Parental Protein 1 |
|---|---|---|---|---|---|---|---|
| A–Black bean | 526 | PVALK | PV, VA, AL, LK | 9.8 | −1 | 15.2 | Photosystem I P700 chlorophyll a apoprotein A2 |
| 565 | DYRL | DY, YR, RL | 6.6 | −2 | 13.0 | Phaseolin alpha-type | |
| 596 | VEGHGV | VE, GH, GV | 5.0 | 1 | 9.6 | Protein kinase PVPK−1 | |
| 650 | FEELN | EE, EL, LN | 2.9 | 0 | 11.3 | Putative resistance protein TIR 17 | |
| 751 | THGPVGAN | TH, HG, GP, GPV, PV, VG, GA | 7.3 | 0 | 10.2 | Nitrate reductase | |
| 757 | ANGSPGGAGA | NG, GS, SP, PG, GG, GA, AG | 5.6 | 0 | 16.1 | Inositol-3-phosphate synthase | |
| 834 | KPSASCSR | KP, KPS, PS, AS | 9.8 | 0 | 18.0 | Glycerol-3-phosphate acyltransferase | |
| 872 | NVGPGSLET | NV, VG, VGP, GP, PG, GS, SL, ET | 3.1 | −1 | 13.8 | Serine/threonine-protein phosphatase PP1 | |
| 983 | PKEDLRLL | PK, KE, LR, LL | 6.9 | 0 | 15.4 | Phaseolin, alpha-type | |
| 983 | PSVADLRLL | PS, SV, VA, AD, LR, LL | 6.7 | 0 | 13.8 | DNA-directed RNA polymerase subunit beta | |
| 1408 | VNPDPAGGPTSGRAL | VN, VNP, NP, DP, PA, AG, GG, GP, PT, TS, SG, GR, RA, AL | 6.7 | 2 | 14.5 | Phaseolin, beta-type | |
| PP–Black bean | 656 | DEGEAH | EG, GE, EA, AH | 3.7 | 0 | 16.4 | Phaseolin, alpha-type |
| 740 | VELVGPK | VE, EL, LV, VG, VGP, GP, PK | 6.5 | −3 | 22.7 | Phaseolin, beta-type | |
| 742 | VELTGPK | VE, LT, LTGP, TGP, TG, GP, PK | 6.5 | 0 | 14.1 | Phaseolin, alpha-type | |
| 850 | SGNGGGGGASM | SG, NG, GG, GA, AS | 5.4 | 1 | 15.3 | Glycine-rich cell wall structural protein | |
| 855 | SKPGGGSPVA | SK, KP, PG, GG, GS, SP, PV, VA | 10.2 | 0 | 13.4 | 9-cis-epoxycarotenoid dioxygenase NCED1 | |
| 943 | KPTTGKGALA | KP, PT, KPT, TT, TG, GK, GK, KG, AL, LA | 10.6 | 2 | 16.1 | 9-cis-epoxycarotenoid dioxygenase NCED1 | |
| A–Green pea | 539 | GPAAGPA | GP, GPA, AA, AG, PA | 5.6 | 1 | 13.29 | Preprotein translocase subunit SECY |
| 541 | TKGGAV | TK, KG, GG, GA, AV | 10.1 | 0 | 11.98 | Aminomethyltransferase, mitochondrial | |
| 543 | NPEGQ | NP, EG, GQ | 3.1 | 0 | 5.7 | Not reported | |
| 544 | TLSPGA | TL, TLS, LSP, SP, PG, GA | 5.3 | −2 | 12.3 | Photosystem II CP43 reaction center protein | |
| PP–Green pea | 620 | SPGDVF | SP, PG, GD, VF | 3.0 | 0 | 10.8 | Mitochondrial Type Ii Peroxiredoxin |
| 627 | LTAVPAG | LT, TA, AV, AVP, VP, PA, AG | 5.5 | −1 | 14.4 | ATP synthase subunit alpha | |
| 678 | HALLLL | HA, AL, LL, LLL | 7.8 | 0 | 11.0 | Photosystem II D2 protein | |
| 683 | SHLGAVT | SH, HL, LG, GA, AV, VT | 7.5 | 0 | 9.1 | Protein translocase subunit SecA, chloroplastic | |
| 687 | GRSAAGVA | GR, AA, AG, GV, VA | 11.1 | 0 | 8.7 | Asparagine synthetase, root | |
| 780 | HSLPGVAT | HS, SL, LP, LPG, PG, GV, VA, AT | 7.5 | −1 | 17.1 | Dihydrolipoyl dehydrogenase, mitochondrial | |
| 785 | RDTAGLGP | TA, AG, GL, LG, LGP, GP | 7.0 | 2 | 15.7 | NAD(P)H-quinone oxidoreductase subunit 5 | |
| A–Chickpea | 856 | DLVLDVPS | LVL, LV, VL, VP, PS | 2.7 | 0 | 15.2 | Tubulin beta chain |
| 943 | KPSSAAGAVR | KP, KPS, PS, AA, AG, GA, AV, VR | 11.5 | −1 | 11.1 | Non-specific lipid-transfer protein | |
| 1091 | TAPHGGLPAGDV | TA, TAP, AP, PH, PHG, GG, GL, LP, PA, GD | 4.9 | 1 | 13.5 | Acidic endochitinase | |
| PP–Chickpea | 428 | SPPE | SP, PP | 3.1 | −1 | 12.4 | Not reported |
| 526 | CSSSSG | SSS, SG | 4.9 | 0 | 10.4 | Alpha-amylase inhibitor | |
| 620 | SPGDV | SP, PG, GD | 3.0 | −1 | 11.1 | Not reported | |
| 812 | TPSGLNPQ | TP, PS, SG, GL, LN, LNP, NP, PQ | 5.2 | 1 | 8.5 | Not reported | |
| 812 | TPEKNPQ | TP, EK, NP, PQ | 6.5 | 0 | 10.8 | Not reported | |
| 815 | EPNGGLVM | EP, PN, NG, GG, GL, LV, VM | 3.0 | 0 | 10.4 | Not reported | |
| 900 | HGAESAGGDT | HG, GA, AE, ES, AG, GG, GD | 3.9 | 0 | 16.4 | Non-specific lipid-transfer protein | |
| 949 | RTPVPPGLL | TP, PV, VP, VPP, PP, PPG, PG, PGL, GL, LL | 11.1 | −1 | 12.2 | Acetyl-coenzyme A carboxylase carboxyl transferase subunit beta | |
| A–Lentil | 467 | VVPGP | VV, VP, PG, PGP, GP | 5.6 | −1 | 10.6 | Not reported |
| 533 | PGDVF | PG, GD, VF | 2.9 | −2 | 12.9 | Not reported | |
| 596 | DGHLR | DG, GH, HL, LR | 7.5 | 0 | 15.5 | Not reported | |
| 652 | EVGTFT | EV, VG, GT, TF, FT | 3.0 | 1 | 13.8 | Not reported | |
| 678 | FEDGLV | DG, DGL, GL, LV | 2.9 | −1 | 11.0 | Not reported | |
| 715 | TPVSAGGK | TP, PV, VS, AG, GG, GK | 9.8 | 0 | 8.4 | Not reported | |
| PP–Lentil | 428 | SPPE | SP, PP | 3.1 | −1 | 12.8 | Not reported |
| 473 | SPGDV | SP, PG, GD | 3.0 | 0 | 8.6 | Not reported | |
| 552 | VPPGAL | VP, VPP, PPG, PP, PG, GA, AL | 5.6 | 1 | 12.6 | Not reported | |
| 627 | LSVPGGV | SV, VP, PG, GG, GGV, GV | 5.5 | 0 | 13.1 | Not reported | |
| 630 | KGGLGVT | KG, GG, GL, LG, LGV, GV, VT | 9.8 | −1 | 13.6 | Not reported | |
| 758 | TSPSPGDV | TS, SP, PS, PG, GD | 3.0 | −1 | 12.2 | Not reported | |
| 942 | KTDVLPTGL | KT, TD, VL, VLP, LP, PT, TG, GL | 6.7 | 0 | 8.1 | Linoleate 9S-lipoxygenase | |
| A–Fava bean | 677 | TPVHPQ | TP, PV, VH, HP, PQ | 7.5 | −1 | 15.2 | Legumin type B alpha chain |
| 682 | NLLAPR | NL, LL, LA, LAP, LLAP, AP, PR | 10.7 | 1 | 8.7 | Probable sucrose-phosphate synthase | |
| 706 | SFGGGGLL | SF, FG, GG, FGG, GL, LL | 5.4 | −1 | 18.3 | 14-3-3-like protein B | |
| PP–Fava bean | 715 | FGGLLPL | FG, FGG, GG, GL, LL, LLP, LP, LPL, PL | 5.4 | 0 | 11.0 | NAD(P)H-quinone oxidoreductase subunit 5 |
| 751 | TKAGGTAF | TK, KA, AG, GG, GT, TA, AF | 9.9 | 0 | 4.8 | 14-3-3-like protein B | |
| 807 | GPPVDVPQ | GP, GPP, PV, VD, VP, PQ | 3.1 | −1 | 12.9 | Photosystem II protein D1 | |
| 810 | PPNGPSEN | PP, PN, NG, GP, PS, SE | 3.0 | 1 | 10.2 | Acid beta-fructofuranosidase | |
| 869 | PPRSDSDP | PP, PR, DP | 3.9 | 1 | 12.7 | Not reported | |
| 942 | PYGVPVGVR | PY, YG, GV, VP, PV, VG, GV, VR | 9.5 | 0 | 8.7 | Elongation factor 1-alpha |
| Black Bean | Binding Affinity (kcal/mol) | Green Pea | Binding Affinity (kcal/mol) | Chickpea | Binding Affinity (kcal/mol) | Lentil | Binding Affinity (kcal/mol) | Fava bean | Binding Affinity (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | HMG | PL | HMG | PL | HMG | PL | HMG | PL | HMG | ||||||
| A | FEELN | −6.1 | −6.7 | GPAAGPA | −7.0 | −7.6 | DLVLDVPS | −6.1 | −6.8 | VVPGP | −7.1 | −7.4 | TPVHPQ | −6.9 | −7.6 |
| THGPVGAN | −6.3 | −7.0 | TKGGAV | −6.0 | −6.8 | KPSSAAGAVR | −6.1 | −6.3 | PGDVF | −7.0 | −7.9 | NLLAPR | −6.3 | −7.6 | |
| ANGSPGGAGA | −6.5 | −8.1 | NPEGQ | −6.3 | −6.6 | TAPHGGLPAGDV | −7.1 | −7.7 | DGHLR | −6.5 | −6.9 | SFGGGGLL | −5.8 | −7.5 | |
| KPSASCSR | −5.9 | −6.7 | TLSPGA | −6.3 | −6.8 | EVGTFT | −7.3 | −8.2 | |||||||
| NVGPGSLET | −6.9 | −7.3 | FEDGLV | −7.6 | −7.0 | ||||||||||
| PKEDLRLL | −5.6 | −7.4 | TPVSAGGK | −6.0 | −6.2 | ||||||||||
| PSVADLRLL | −5.9 | −7.7 | SPPE | −6.6 | −7.7 | ||||||||||
| VNPDPAGGPTSGRAL | −7.0 | −8.0 | SPGDVF | −6.7 | −7.3 | CSSSSG | −5.5 | −6.1 | SPPE | −6.8 | −7.5 | ||||
| PP | DEGEAH | −6.3 | −7.6 | LTAVPAG | −6.3 | −6.3 | SPGDV | −7.0 | −6.8 | SPGDV | −6.4 | −6.6 | FGGLLPL | −6.6 | −7.6 |
| VELVGPK | −6.2 | −6.9 | HALLLL | −5.9 | −6.9 | TPSGLNPQ | −6.9 | −7.8 | VPPGAL | −6.4 | −7.7 | TKAGGTAF | −5.5 | −7.2 | |
| VELTGPK | −6.4 | −6.7 | SHLGAVT | −6.5 | −6.7 | TPEKNPQ | −6.9 | −7.1 | LSVPGGV | −6.2 | −7.2 | GPPVDVPQ | −5.8 | −6.1 | |
| SGNGGGGGASM | −6.0 | −6.8 | GRSAAGVA | −5.8 | −6.7 | EPNGGLVM | −5.7 | −7.0 | KGGLGVT | −6.4 | −6.7 | PPNGPSEN | −5.9 | −8.1 | |
| SKPGGGSPVA | −5.6 | −7.9 | HSLPGVAT | −6.8 | −7.1 | HGAESAGGDT | −5.8 | −6.8 | TSPSPGDV | −7.0 | −7.4 | PPRSDSDP | −7 | −7.7 | |
| KPTTGKGALA | −6.4 | −7.0 | RDTAGLGP | −6.5 | −7.3 | RTPVPPGLL | −6.8 | −7.9 | KTDVLPTGL | −6.3 | −6.7 | PYGVPVGVR | −6.9 | −8.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, C.; Mojica, L.; González de Mejía, E.; Camacho Ruiz, R.M.; Luna-Vital, D.A. Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis. Foods 2020, 9, 1678. https://doi.org/10.3390/foods9111678
Moreno C, Mojica L, González de Mejía E, Camacho Ruiz RM, Luna-Vital DA. Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis. Foods. 2020; 9(11):1678. https://doi.org/10.3390/foods9111678
Chicago/Turabian StyleMoreno, Cecilia, Luis Mojica, Elvira González de Mejía, Rosa María Camacho Ruiz, and Diego A. Luna-Vital. 2020. "Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis" Foods 9, no. 11: 1678. https://doi.org/10.3390/foods9111678
APA StyleMoreno, C., Mojica, L., González de Mejía, E., Camacho Ruiz, R. M., & Luna-Vital, D. A. (2020). Combinations of Legume Protein Hydrolysates Synergistically Inhibit Biological Markers Associated with Adipogenesis. Foods, 9(11), 1678. https://doi.org/10.3390/foods9111678








