Optimization and Modeling of Slightly Acidic Electrolyzed Water for the Clean-in-Place Process in Milking Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Milk Preparation
2.3. Specimens Preparation
2.4. Response Surface Design and Validation
2.5. Preparation of SAEW and Chemical Disinfectant
2.6. Disinfectant Treatments
2.7. Bacterial Counting and Cleanliness Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Treatment Solutions
3.2. SAEW Cleaning Efficiency in Removing Bacteria and ATP
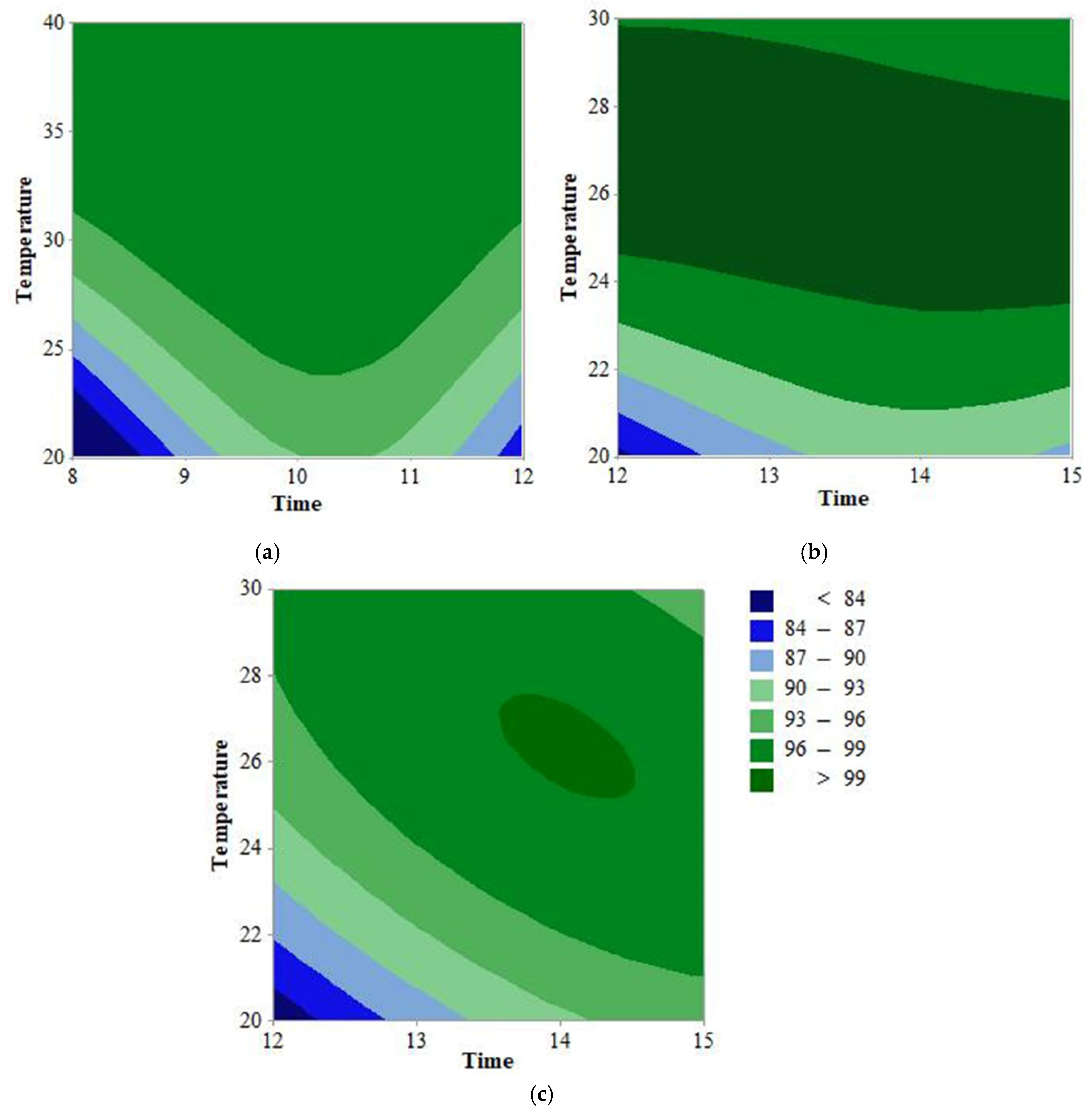
3.3. Model Fitting
3.4. Validation of the Models
3.5. Optimization and Validation of SAEW Cleaning Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DPC. Guidelines for Installation, Cleaning, and Sanitizing of Large and Multiple Receiver Parlor Milking Systems; Dairy Practices Council Publication: Pandora, OH, USA, 2010. [Google Scholar]
- Dev, S.R.S.; Demirci, A.; Graves, R.E.; Puri, V.M. Optimization and modeling of an electrolyzed oxidizing water based Clean-In-Place technique for farm milking systems using a pilot-scale milking system. J. Food. Eng. 2014, 135, 1–10. [Google Scholar] [CrossRef]
- HICAHS. The High Plains Intermountain Center for Agricultural Health and Safety—Factsheets, Colorado State University. Children on the Farm. Available online: http://www.hicahs.colostate.edu (accessed on 15 June 2012).
- Jiménez-Pichardo, R.; Regalado, C.; Castaño-Tostado, E.; Meas-Vong, Y.; Santos-Cruz, J.; García-Almendárez, B.E. Evaluation of electrolyzed water as cleaning and disinfection agent on stainless steel as a model surface in the dairy industry. Food Control. 2016, 60, 320–328. [Google Scholar] [CrossRef]
- Walker, S.P.; Demirci, A.; Graves, R.E.; Spencer, S.B.; Roberts, R.F. Cleaning milking systems using electrolyzed oxidizing water. Trans. ASAE 2005, 48, 1827–1833. [Google Scholar] [CrossRef]
- Davey, K.R.; Chandrakash, S.; O’Neill, B.K. A new risk analysis of clean-in-place milk processing. Food Control. 2013, 29, 248–253. [Google Scholar] [CrossRef]
- Suárez, L.; Díez, M.A.; García, R.; Riera, F.A. Membrane technology for the recovery of detergent compounds: A review. J. Ind. Eng. Chem. 2012, 18, 1859–1873. [Google Scholar] [CrossRef]
- Vilar, M.J.; Rodríguez-Otero, J.L.; Diéguez, F.J.; Sanjuán, M.L.; Yus, E. Application of ATP bioluminescence for evaluation of surface cleanliness of milking equipment. Int. J. Food. Microbiol. 2008, 125, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.Y.; Shi, Z.X.; Li, B.M. Disinfection effect and cleaning mode determination for milk tank using electrolyzed water. Trans. CSAE. 2017, 33, 300–306, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, X.J.; Cao, H.F.; Qi, X.M.; Wang, C.Y.; Liu, H.F.; Yun, Z.Y. Research progress of cleaning sanitization methods and effect detection for dairy production equipment. China Dairy Ind. 2015, 43, 49–51, (In Chinese with English Abstract). [Google Scholar]
- Suzuki, T.; Itakura, J.; Watanabe, M.; Ohta, M.; Sato, Y.; Yamaya, Y. Inactivation of Staphylococcal enterotoxin-A with an electrolyzed anodic solution. J. Agric. Food. Chem. 2002, 50, 230–234. [Google Scholar] [CrossRef]
- Suzuki, T.; Noro, T.; Kawamura, Y.; Fukunaga, K.; Watanabe, M.; Ohta, M.; Sugiue, H.; Sato, Y.; Kohno, M.; Hotta, K. Decontamination of aflatoxin-forming fungus and elimination of aflatoxin mutagenicity with electrolyzed NaCl anode solution. J. Agric. Food. Chem. 2002, 50, 633–641. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs, U.S. Environmental Protection Agency; U.S. Environmental Protection Agency, Office of Pesticide Programs: Washington, DC, USA, 2004.
- Kim, C.; Hung, Y.; Brackett, R.E. Roles of oxidation–reduction potential in electrolyzed oxidizing and chemically modified water for inactivation of food related pathogens. J. Food. Protect. 2000, 63, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Demirci, A.; Graves, R.E.; Spencer, S.B.; Roberts, R.F. Response surface modelling for cleaning and disinfecting materials used in milking systems with electrolyzed oxidizing water. Int. J. Dairy. Technol. 2005, 58, 65–73. [Google Scholar] [CrossRef]
- Wang, X.M.; Demirci, A.; Puri, V.M.; Graves, R.E. Evaluation of blended electrolyzed oxidizing water-based cleaning-in-place (CIP) technique using a laboratory-scale milking system. Trans. ASABE 2016, 59, 359–370. [Google Scholar] [CrossRef]
- Wang, X.M.; Puri, V.M.; Demirci, A.; Graves, R.E. One-step cleaning-in-place for milking systems and mathematical modeling for deposit removal from stainless steel pipeline using blended electroloyzed oxidizing water. Trans. ASABE 2016, 59, 1893–1904. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.Y.; Shi, Z.X.; Li, B.M. Cleaning and bacteria removal in milking systems by alkaline electrolyzed oxidizing water with response surface design. Trans. ASABE 2019, 62, 1251–1258. [Google Scholar] [CrossRef]
- Gil, M.I.; Gómez-López, V.M.; Hung, Y.C.; Allende, A. Potential of electrolyzed water as an alternative disinfectant agent in the fresh-cut industry. Food. Bioprocess. Tech. 2015, 8, 1336–1348. [Google Scholar] [CrossRef]
- Ayebah, B.; Hung, Y.C. Electrolyzed water and its corrosiveness on various surface materials commonly found in food processing facilities. J. Food. Process. Eng. 2005, 28, 247–264. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Vinas, I. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally processed vegetables. Int. J. Food. Microbiol. 2008, 31, 121–129. [Google Scholar] [CrossRef]
- Hao, J.X.; Liu, H.; Liu, R.U.I.; Dalai, W.; Zhao, R.; Chen, T.; Li, L.T. Efficacy of slightly acidic electrolyzed water (SAEW) for reducing microbial contamination on fresh-cut cilantro. J. Food. Safety. 2010, 31, 28–34. [Google Scholar] [CrossRef]
- Issa-Zacharia, A.; Kamitani, Y.; Morita, K.; Wasaki, K. Sanitization potency of slightly acidic electrolyzed water against pure cultures of Escherichia coli and Staphylococcus aureus, in comparison with that of other food sanitizers. Food Control. 2010, 21, 740–745. [Google Scholar] [CrossRef]
- Aycicek, H.; Oguz, U.; Karci, K. Comparison of results of ATP bioluminescence and traditional hygiene swabbing methods for the determination of surface cleanliness at a hospital kitchen. Int. J. Hyg. Environ. Health 2006, 209, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Samkutty, P.J.; Gough, R.H.; Adkinson, R.W.; McGrew, P. Rapid assessment of the bacteriological quality of raw milk using ATP bioluminescence. J. Food. Protec. 2001, 64, 208–212. [Google Scholar] [CrossRef] [PubMed]
- NHFPC. Food Enterprise HACCP Implementation Guide; NHFPC: Beijing, China, 2006. Available online: https://www.jdzx.net.cn/article/402881e40c5730e0010c86c90f25006b/2015/11/402881e40c5730e0010c86e3c1be0087.html (accessed on 19 June 2006). (In Chinese)
- Bremer, P.J.; Monk, I.; Butler, R. Inactivation of Listeria monocytogenes/ Flavobacterium spp. biofilm using chlorine: Impact of substrate, pH, time and concentration. Lett. Appl. Microbiol. 2002, 35, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Flint, S.H.; Brooks, J.D. Evaluation of the effect of cleaning regimes on biofilms of thermophilic bacilli on stainless steel. J. Appl. Microbiol. 2004, 96, 110–116. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Food Code; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2005.
- Mokgatla, R.M.; Gouws, P.A.; Brozel, V.S. Mechanisms contributing to hypochlorous acid resistance of a Salmonella isolate from a poultry-processing plant. J. Appl. Microbiol. 2002, 92, 566–573. [Google Scholar] [CrossRef]
- Chen, G.Q.; Li, A.B.; Liang, J.J.; Zhou, S.H.; Zhu, Y.K.; Huang, W.; Yu, Y. Experimental study on germicidal efficacy and its influencing factors of electrolyzed oxidizing water. Pract. Prev. Med. 2001, 8, 269–271, (In Chinese with English Abstract). [Google Scholar]
- Zang, Y.T.; Li, B.M.; Bing, S.; Cao, W. Modeling disinfection of plastic poultry transport cages inoculated with Salmonella enteritids by slightly acidic electrolyzed water using response surface methodology. Poultry. Sci. 2015, 94, 2059–2065. [Google Scholar] [CrossRef]
- Ni, L.; Cao, W.; Zheng, W.C.; Zhang, Q.; Li, B.M. Reduction of microbial contamination on the surfaces of layer houses using slightly acidic electrolyzed water. Poultry. Sci. 2015, 94, 2838–2848. [Google Scholar] [CrossRef]
- Davidson, C.A.; Griffith, C.J.; Peters, A.C.; Fielding, L.M. Evaluation of two methods for monitoring surface cleanliness-ATP bioluminescence and traditional hygiene swabbing. Luminescence 1999, 14, 33–38. [Google Scholar] [CrossRef]
- Latorre, A.A.; Van Kessel, J.S.; Karns, J.S.; Zurakowski, M.J.; Pradhan, A.K.; Boor, K.J.; Jayarao, B.M.; Houser, B.A.; Daugherty, C.S.; Schukken, Y.H. Biofilm in milking equipment on a dairy farm as a potential source of bulk tank milk contamination with Listeria monocytogenes. J. Dairy. Sci. 2010, 93, 2792–2802. [Google Scholar] [CrossRef]


| Trial | Time (min) | Temperature (°C) | ACC (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SS 1 | RG | PVC | SS | RG | PVC | SS | RG | PVC | |
| 1 | 10 | 15 | 15 | 40 | 30 | 30 | 40 | 50 | 50 |
| 2 | 8 | 15 | 15 | 30 | 25 | 25 | 40 | 60 | 60 |
| 3 | 10 | 12 | 12 | 30 | 20 | 20 | 50 | 50 | 50 |
| 4 | 8 | 12 | 12 | 40 | 25 | 25 | 50 | 60 | 60 |
| 5 | 8 | 15 | 15 | 30 | 25 | 25 | 60 | 40 | 40 |
| 6 | 8 | 13.5 | 13.5 | 20 | 25 | 25 | 50 | 50 | 50 |
| 7 | 12 | 12 | 12 | 30 | 30 | 30 | 60 | 50 | 50 |
| 8 | 12 | 13.5 | 13.5 | 40 | 30 | 30 | 50 | 40 | 40 |
| 9 | 10 | 15 | 15 | 40 | 20 | 20 | 60 | 50 | 50 |
| 10 | 10 | 13.5 | 13.5 | 30 | 20 | 20 | 50 | 40 | 40 |
| 11 | 12 | 13.5 | 13.5 | 30 | 25 | 25 | 40 | 50 | 50 |
| 12 | 10 | 13.5 | 13.5 | 20 | 20 | 20 | 40 | 60 | 60 |
| 13 | 12 | 12 | 12 | 20 | 25 | 25 | 50 | 40 | 40 |
| 14 | 10 | 13.5 | 13.5 | 20 | 30 | 30 | 60 | 60 | 60 |
| 15 | 10 | 13.5 | 13.5 | 30 | 25 | 25 | 50 | 50 | 50 |
| Trial | Treatment Time (min) | Cleaning Temperature (°C) | ACC (mg/L) |
|---|---|---|---|
| 1 | 9 | 20 | 40 |
| 2 | 9 | 30 | 50 |
| 3 | 9 | 40 | 60 |
| 4 | 11 | 20 | 60 |
| 5 | 11 | 30 | 50 |
| 6 | 11 | 40 | 40 |
| Solutions | ACC (mg/L) | pH | ORP (mV) |
|---|---|---|---|
| Tap water | 0 ± 0 | 7.65 ± 0.01 | 402 ± 3 |
| SAEW | 18 ± 1 | 6.25 ± 0.04 | 732 ± 9 |
| 30 ± 1 | 6.30 ± 0.07 | 814 ± 4 | |
| 42 ± 1 | 5.88 ± 0.05 | 904 ± 7 | |
| 53 ± 0 | 6.25 ± 0.11 | 928 ± 5 | |
| 62 ± 2 | 6.00 ± 0.02 | 924 ± 8 | |
| Super | 0 ± 0 | 1.61 ± 0.01 | 692 ± 1 |
| Solution | Trial | Removing Bacteria (log10 CFU/cm2) | Removing ATP (RLU) | ||||
|---|---|---|---|---|---|---|---|
| SS 1 | RG | PVC | SS | RG | PVC | ||
| SAEW | 1 | 6.00 ± 0.00 *b 2 | 5.45 ± 0.00 *a | 5.64 ± 0.00 *b | 265 ± 0.00 b | 130 ± 2.12 b | 420 ± 0.71 b |
| 2 | 2.86 ± 0.90 b 3 | 4.23 ± 0.00 b | 3.18 ± 0.00 *a | 225 ± 0.71 b | 231 ± 5.66 b | 405 ± 0.71 b | |
| 3 | 4.67 ± 0.38 a | 1.59 ± 0.34 b | 4.18 ± 1.07 b | 365 ± 0.00 b | 115 ± 0.00 b | 360 ± 2.12 b | |
| 4 | 6.00 ± 0.00 *b | 1.79 ± 0.01 b | 3.18 ± 0.00 *a | 265 ± 0.00 b | 224 ± 7.78 b | 399 ± 0.71 b | |
| 5 | 6.15 ± 0.00 *b | 5.81 ± 0.00 *b | 3.95 ± 0.00 *b | 268 ± 1.41 b | 225 ± 0.71 b | 415 ± 2.83 b | |
| 6 | 2.50 ± 0.01 b | 2.11 ± 0.46 b | 3.02 ± 0.34 a | 120 ± 0.71 b | 170 ± 0.71 b | 415 ± 1.41 b | |
| 7 | 6.15 ± 0.00 *b | 4.57 ± 1.75 a | 3.26 ± 0.00 a | 263 ± 8.49 b | 220 ± 2.83 a | 410 ± 2.12 b | |
| 8 | 6.00 ± 0.00 *b | 5.56 ± 0.00 *a | 3.98 ± 0.00 *b | 270 ± 0.00 b | 185 ± 0.71 b | 325 ± 1.41 b | |
| 9 | 6.00 ± 0.00 *b | 3.91 ± 0.51 b | 4.29 ± 1.91 b | 265 ± 0.00 b | 125 ± 0.71 b | 400 ± 0.00 b | |
| 10 | 4.67 ± 0.38 a | 2.94 ± 0.51 b | 3.98 ± 0.00 *b | 355 ± 2.12 *b | 165 ± 0.00 b | 310 ± 1.41 b | |
| 11 | 4.60 ± 0.71 a | 5.81 ± 0.00 *b | 3.95 ± 0.00 *b | 230 ± 0.00 b | 230 ± 0.71 b | 455 ± 0.71 b | |
| 12 | 2.95 ± 1.19 b | 5.44 ± 0.70 a | 4.29 ± 0.00 *b | 215 ± 0.00 b | 275 ± 0.00 b | 303 ± 0.71 b | |
| 13 | 2.58 ± 0.44 b | 3.05 ± 0.91 b | 3.00 ± 0.00 *a | 130 ± 0.71 b | 170 ± 0.71 b | 245 ± 0.00 b | |
| 14 | 4.48 ± 2.36 a | 5.94 ± 0.00 *b | 4.29 ± 0.00 *b | 267 ± 0.71 b | 285 ± 0.71 b | 313 ± 2.12 b | |
| 15 | 5.59 ± 0.49 b | 5.46 ± 0.50 a | 2.00 ± 1.41 b | 360 ± 1.41 b | 220 ± 2.12 a | 383 ± 1.41 b | |
| Super | 4.18 ± 1.15 a | 5.06 ± 0.49 a | 2.83 ± 0.50 a | 92 ± 15.56 a | 219 ± 5.66 a | 392 ± 19.09 a | |
| Materials | Removal Efficiency 1 | Models 2 | R2 | p | Lack of Fit |
|---|---|---|---|---|---|
| Stainless steel | log10 bacterial reduction | RS = −6.240 + 0.802x1 + 0.155x2 + 0.040x3 − 0.006x1x3 − 0.001x2x3 − 0.024x12 − 0.001x22 + 0.001x32 | 0.90 | 0.05 | 0.24 |
| ATP removal rate | RS-ATP = −84.900 + 23.800x1 + 3.590x2–1.153x12 − 0.051x22 | 0.70 | 0.01 | 0.14 | |
| Rubber gasket | log10 bacterial reduction | RR = −86.500 + 12.120x1 + 0.191x2 − 0.423x12 | 0.56 | 0.03 | 0.97 |
| ATP removal rate | RR-ATP = −329 + 27.200x1 + 21.950x2 − 1.840x3 − 0.381x1x2 + 0.082x1x3 − 0.034x2x3 − 0.787x12–0.285x22 + 0.016x32 | 0.94 | 0.01 | 0.57 | |
| PVC | log10 bacterial reduction | RP = 51.000 − 1.605x1 − 3.123x2 + 0.076x1x2 + 0.042x22 | 0.70 | 0.01 | 0.99 |
| ATP removal rate | RP-ATP = −411 + 42.500x1 + 15.500x2 + 0.153x3 − 0.488x1x2 − 1.055x12 − 0.164x22 | 0.85 | 0.01 | 0.05 |
| Material | Treatment Time (min) | Cleaning Temperature (°C) | ACC (mg/L) |
|---|---|---|---|
| Stainless steel pipe | 9.9 | 37.8 | 60 |
| Rubber gasket | 13.8 | 28.5 | 40 |
| PVC milk hose | 14.9 | 30 | 60 |
| Combining rubber and PVC | 14.4 | 29.6 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, C.; Shi, Z.; Li, B. Optimization and Modeling of Slightly Acidic Electrolyzed Water for the Clean-in-Place Process in Milking Systems. Foods 2020, 9, 1685. https://doi.org/10.3390/foods9111685
Liu Y, Wang C, Shi Z, Li B. Optimization and Modeling of Slightly Acidic Electrolyzed Water for the Clean-in-Place Process in Milking Systems. Foods. 2020; 9(11):1685. https://doi.org/10.3390/foods9111685
Chicago/Turabian StyleLiu, Yu, Chaoyuan Wang, Zhengxiang Shi, and Baoming Li. 2020. "Optimization and Modeling of Slightly Acidic Electrolyzed Water for the Clean-in-Place Process in Milking Systems" Foods 9, no. 11: 1685. https://doi.org/10.3390/foods9111685
APA StyleLiu, Y., Wang, C., Shi, Z., & Li, B. (2020). Optimization and Modeling of Slightly Acidic Electrolyzed Water for the Clean-in-Place Process in Milking Systems. Foods, 9(11), 1685. https://doi.org/10.3390/foods9111685






