Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Enterobacteriaceae Colony-Forming Unit Count
2.3. Isolation and Identification of Enterobacterales
2.4. Antimicrobial Susceptibility Testing
2.5. Genomic DNA Extraction and Polymerase Chain Reaction (PCR) Amplification
2.6. Statistical Analysis
3. Results
3.1. Bacterial Diversity
3.2. Enterobacteriaceae Colony-Forming Unit (CFU) Count
3.3. Identification and Prevalence of β-Lactamase-Producing Enterobacterales
3.4. Antibiotic Resistance Genotype Profile
3.5. Antibiotic Susceptibility Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- ECDC; EFSA; EMA. First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2015, 13, 4006. [Google Scholar] [CrossRef]
- Valentin, L.; Sharp, H.; Hille, K.; Seibt, U.; Fischer, J.; Pfeifer, Y.; Michael, G.B.; Renne Nickel, S.; Schmiedel, J.; Falgenhauer, L.; et al. Subgrouping of ESBL-producing Escherichia coli from animal and human sources: An approach to quantify the distribution of ESBL types between different reservoirs. Int. J. Med. Microbiol. 2014, 304, 805–816. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40. [Google Scholar] [CrossRef] [PubMed]
- Blaak, H.; van Hoek, A.H.A.M.; Veenman, C.; Docters van Leeuwen, A.E.; Lynch, G.; van Overbeek, W.M.; de Roda Husman, A.M. Extended spectrum β-lactamase- and constitutively AmpC-producing Enterobacteriaceae on fresh produce and in the agricultural environment. Int. J. Food Microbiol. 2014, 168–169, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Regulation No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R1831&rid=10 (accessed on 14 July 2020).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2016—Trends from 2010 to 2016; Eight ESVAC Report; European Medicines Agency: London, UK, 2018; Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-30-european-countries-2016-trends-2010-2016-eighth-esvac_en.pdf (accessed on 14 July 2020).
- Van Hoek, A.H.A.M.; Veenman, C.; van Overbeek, W.M.; Lynch, G.; de Roda Husman, A.M.; Blaak, H. Prevalence and characterization of ESBL- and AmpC-producing Enterobacteriaceae on retail vegetables. Int. J. Food Microbiol. 2015, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. European Centre for Disease Prevention and Control Multi-country outbreak of Listeria monocytogenes serogroup IVb, multi-locus sequence type 6, infections linked to frozen corn and possibly to other frozen vegetables—First update. EFSA Supporting Publ. 2018, 15. [Google Scholar] [CrossRef]
- Outbreak of Salmonella Newport Infections Linked to Onions. Available online: https://www.cdc.gov/salmonella/newport-07-20/updates.html (accessed on 5 November 2020).
- CDC Antibiotic Resistant Threat Report. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 5 November 2020).
- Point Prevalence Survey Database (HAI-Net). Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections-acute-care-hospitals/surveillance-disease-data/database (accessed on 8 August 2020).
- Schwaber, M.J.; Carmeli, Y. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 60, 913–920. [Google Scholar] [CrossRef]
- Carattoli, A. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 2008, 14. [Google Scholar] [CrossRef]
- Randall, L.P.; Lodge, M.P.; Elviss, N.C.; Lemma, F.L.; Hopkins, K.L.; Teale, C.J.; Woodford, N. Evaluation of meat, fruit and vegetables from retail stores in five United Kingdom regions as sources of extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Escherichia coli. Int. J. Food Microbiol. 2017, 241, 283–290. [Google Scholar] [CrossRef]
- Ruimy, R.; Brisabois, A.; Bernede, C.; Skurnik, D.; Barnat, S.; Arlet, G.; Momcilovic, S.; Elbaz, S.; Moury, F.; Vibet, M.A.; et al. Organic and conventional fruits and vegetables contain equivalent counts of Gram-negative bacteria expressing resistance to antibacterial agents. Environ. Microbiol. 2010, 12, 608–615. [Google Scholar] [CrossRef]
- European Antimicrobial Resistance Surveillance Network (EARS-Net). Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net (accessed on 8 August 2020).
- European Centre for Disease Prevention and Control. Country Visit to Romania to Discuss. Antimicrobial Resistance Issues; Mission Report; ECDC: Solna, Sweden, 2018. [Google Scholar]
- Hölzel, C.S.; Tetens, J.L.; Schwaiger, K. Unraveling the role of vegetables in spreading antimicrobial-resistant bacteria: A need for quantitative risk assessment. Foodborne Pathog. Dis. 2018, 15, 671–688. [Google Scholar] [CrossRef]
- Martinez-Martinez, L.; Cantón Spain, R.; Stefani, S.; Skov, R.; Glupczynski, Y.; Nordmann, P.; Wootton, M.; Miriagou, V.; Skov Simonsen, G. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance; EUCAST: Växjö, Sweden, 2017. [Google Scholar]
- M100Ed30—Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 8 August 2020).
- Kerckhof, F.M.; Courtens, E.N.P.; Geirnaert, A.; Hoefman, S.; Ho, A.; Vilchez-Vargas, R.; Pieper, D.H.; Jauregui, R.; Vlaeminck, S.E.; Van De Wiele, T.; et al. Optimized cryopreservation of mixed microbial communities for conserved functionality and diversity. PLoS ONE 2014, 9, e99517. [Google Scholar] [CrossRef]
- Reuland, E.A.; al Naiemi, N.; Raadsen, S.A.; Savelkoul, P.H.M.; Kluytmans, J.A.J.W.; Vandenbroucke-Grauls, C.M.J.E. Prevalence of ESBL-producing Enterobacteriaceae in raw vegetables. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Dallenne, C.; da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Nüesch-Inderbinen, M.; Morach, M.; Berner, A.Z.; Hächler, H.; Stephan, R. Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 2015, 81, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Du Plessis, E.M.; Duvenage, S.; Korsten, L. Occurrence, Identification, and Antimicrobial Resistance Profiles of Extended-Spectrum and AmpC β-Lactamase-Producing Enterobacteriaceae from Fresh Vegetables Retailed in Gauteng Province, South Africa. Foodborne Pathog. Dis. 2019, 16, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.T.; Zhang, X.Y.; Wan, S.W.; Hao, J.J.; Jiang, R.; De Song, F.J. Characteristics of carbapenem-resistant Enterobacteriaceae in ready-to-eat vegetables in China. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Raphael, E.; Wong, L.K.; Riley, L.W. Extended-spectrum beta-lactamase gene sequences in Gram-negative saprophytes on retail organic and nonorganic spinach. Appl. Environ. Microbiol. 2011, 77, 1601–1607. [Google Scholar] [CrossRef]
- Jung, D.; Rubin, J.E. Identification of antimicrobial resistant bacteria from plant-based food products imported into Canada. Int. J. Food Microbiol. 2020, 319, 108509. [Google Scholar] [CrossRef]
- Sapkota, S.; Adhikari, S.; Khadka, S.; Adhikari, M.; Kandel, H.; Pathak, S.; Pandey, A.; Pandey, A. Multi-drug resistant extended-spectrum beta-lactamase producing E. coli and Salmonella on raw vegetable salads served at hotels and restaurants in Bharatpur, Nepal. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Tarco, E.; Butiuc-Keul, A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. Germs 2019, 9, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, L.; Jouini, A.; Klibi, N.; Dziri, R.; Alonso, C.A.; Boudabous, A.; Ben Slama, K.; Torres, C. Detection of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in vegetables, soil and water of the farm environment in Tunisia. Int. J. Food Microbiol. 2015, 203, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, Y.; Cullik, A.; Witte, W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int. J. Med. Microbiol. 2010, 300. [Google Scholar] [CrossRef]
- Pitout, J.D.D. Extraintestinal pathogenic Escherichia coli: An update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev. Anti. Infect. Ther. 2012, 10. [Google Scholar] [CrossRef]
- Liebana, E.; Carattoli, A.; Coque, T.M.; Hasman, H.; Magiorakos, A.P.; Mevius, D.; Peixe, L.; Poirel, L.; Schuepbach-Regula, G.; Torneke, K.; et al. Public health risks of enterobacterial isolates producing extended-spectrum β-lactamases or AmpC β-lactamases in food and food-producing animals: An EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin. Infect. Dis. 2013, 56, 1030–1037. [Google Scholar] [CrossRef]
- Maciuca, I.E.; Williams, N.J.; Tuchilus, C.; Dorneanu, O.; Guguianu, E.; Carp-Carare, C.; Rimbu, C.; Timofte, D. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 2015, 21, 651–662. [Google Scholar] [CrossRef]
- Iseppi, R.; De Niederhaüsern, S.; Bondi, M.; Messi, P.; Sabia, C. Extended-spectrum β-lactamase, AmpC, and MBL-producing gram-negative bacteria on fresh vegetables and ready-to-eat salads sold in local markets. Microb. Drug Resist. 2018, 24, 1156–1164. [Google Scholar] [CrossRef]
- Kaesbohrer, A.; Bakran-Lebl, K.; Irrgang, A.; Fischer, J.; Kämpf, P.; Schiffmann, A.; Werckenthin, C.; Busch, M.; Kreienbrock, L.; Hille, K. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet. Microbiol. 2019, 233, 52–60. [Google Scholar] [CrossRef]
- Kawamura, K.; Goto, K.; Nakane, K.; Arakawa, Y. Molecular epidemiology of extended-spectrum β-lactamases and Escherichia coli isolated from retail foods including chicken meat in Japan. Foodborne Pathog. Dis. 2014, 11, 104–110. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Buys, E.M. Pathogenic and commensal Escherichia coli from irrigation water show potential in transmission of extended spectrum and AmpC β-lactamases determinants to isolates from lettuce. Microb. Biotechnol. 2015, 8, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Ozeki, K.; Komatsu, T.; Fukuda, A.; Tamura, Y. Prevalence of extended-spectrum b-lactamase–producing bacteria on fresh vegetables in Japan. J. Food Prot. 2019, 82, 1663–1666. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhang, S.; Zhang, J.; Yang, G.; Wang, J.; Xue, L.; Chen, M. Characterization of extended-spectrum β-lactamase-producing Enterobacteriaceae from retail food in China. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khari, F.I.M.; Karunakaran, R.; Rosli, R.; Tay, S.T. Genotypic and phenotypic detection of AmpC β-lactamases in Enterobacter spp. Isolated from a teaching hospital in Malaysia. PLoS ONE 2016, 11, e0150643. [Google Scholar] [CrossRef]
- Lixandru, B.E.; Cotar, A.I.; Straut, M.; Usein, C.R.; Cristea, D.; Ciontea, S.; Tatu-Chitoiu, D.; Codita, I.; Rafila, A.; Nica, M.; et al. Carbapenemase-producing Klebsiella pneumoniae in Romania: A six-month survey. PLoS ONE 2015, 10, e0143214, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.J.; Rubin, J.E. Carbapenemase producing bacteria in the food supply escaping detection. PLoS ONE 2015, 10, e0126717. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017, 2. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A.; Baloul, Y.; Lalaoui, R.; Bakour, S.; Thighilt, L.; Gharout, A.; Rolain, J.M. First detection of Klebsiella pneumoniae producing OXA-48 in fresh vegetables from Béjaïa city. Algeria. J. Glob. Antimicrob. Resist. 2017, 9, 17–18. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Luo, J.; Lv, L.; Zeng, Z.; Liu, J.H. Emergence of Escherichia coli coproducing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J. Antimicrob. Chemother. 2018, 73, 252–254. [Google Scholar] [CrossRef]
- Bolton, E. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat. Foods Placed on the Market; Health Protection Agency: London, UK, 2009; p. 33. [Google Scholar]
- Uhlig, E.; Olsson, C.; He, J.; Stark, T.; Sadowska, Z.; Molin, G.; Ahrné, S.; Alsanius, B.; Håkansson, Å. Effects of household washing on bacterial load and removal of Escherichia coli from lettuce and “ready-to-eat” salads. Food Sci. Nutr. 2017, 5, 1215–1220. [Google Scholar] [CrossRef]
- Xylia, P.; Botsaris, G.; Chrysargyris, A.; Skandamis, P.; Tzortzakis, N. Variation of microbial load and biochemical activity of ready-to-eat salads in Cyprus as affected by vegetable type, season, and producer. Food Microbiol. 2019, 83, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C.D. Understanding the routes of contamination of ready-to-eat vegetables in the Middle East. Food Control 2016, 62, 125–133. [Google Scholar] [CrossRef]
- Davidson, P.M.; Taylor, T.M. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2007; pp. 765–801. [Google Scholar] [CrossRef]
- Solomon, E.B.; Matthews, K.R. Use of fluorescent microspheres as a tool to investigate bacterial interactions with growing plants. J. Food Prot. 2005, 68, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible Coatings Containing Oregano Essential Oil Nanoemulsion for Improving Postharvest Quality and Shelf Life of Tomatoes. Foods 2020, 9, E1605. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 10, 106–120. [Google Scholar] [CrossRef]

| Beta-lactamase (s) Targeted | Primer Sequence For/Rev | Length (Bases) | Primer Concentration (pmol/µL) | Amplicon Size (bp) | Annealing Position * |
|---|---|---|---|---|---|
| TEM variants, including TEM-1 and TEM-2 | For CATTTCCGTGTCGCCCTTATTC | 22 | 0.4 | 800 | 13–34 |
| Rev CGTTCATCCATAGTTGCCTGAC | 22 | 0.4 | 812–791 | ||
| SHV-1 and variants | For AGCCGCTTGAGCAAATTAAAC | 21 | 0.4 | 713 | 71–91 |
| Rev ATCCCGCAGTAAATCACCAC | 21 | 0.4 | 783–763 | ||
| OXA-1, OXA-4 and OXA-30 | For GGCACCAGATTCAACTTTCAAG | 22 | 0.4 | 564 | 201–222 |
| Rev GACCCCAAGTTTCCTGTAAGTG | 22 | 0.4 | 764–743 | ||
| CTX-M-1, CTX-M-3 and CTX-M-15 | For TTAGGAAATGTGCCGCTGTA | 20 | 0.4 | 688 | 61–80 |
| Rev CGATATCGTTGGTGGTACCAT | 21 | 0.4 | 748–728 | ||
| CMY-1, CMY-8, CMY-11, CMY-19 and MOX-1, MOX-2 | For GCAACAACGACAATCCATCCT | 21 | 0.2 | 895 | 3–23 |
| Rev GGGATAGGCGTASCTCTCCCAA | 22 | 0.2 | 900–879 | ||
| DHA-1 and DHA-2 | For TGATGGCACAGCAGGATATTC | 21 | 0.5 | 997 | 113–133 |
| Rev GCTTTGACTCTTTCGGTATTCG | 22 | 0.5 | 1109–1088 | ||
| VEB-1 to VEB-6 | For CATTTCCCGATGCAAAGCGT | 20 | 0.3 | 648 | 187–206 |
| Rev CGAAGTTTCTTTGGACTCTG | 20 | 0.3 | 834–815 | ||
| IMP variants except IMP-9, IMP-16, IMP-18, IMP-22 and IMP-25 | For TGACACTCCATTTACAG | 18 | 0.5 | 139 | 194–211 |
| Rev GATTGAGAATTAAGCCACCCT | 21 | 0.5 | 332–313 | ||
| KPC-1 to KPC-5 | For CATTCAAGGGCTTTCTTGCTGC | 22 | 0.2 | 538 | 209–230 |
| Rev ACGACGGCATAGTCATCATTTGC | 20 | 0.2 | 746–272 |
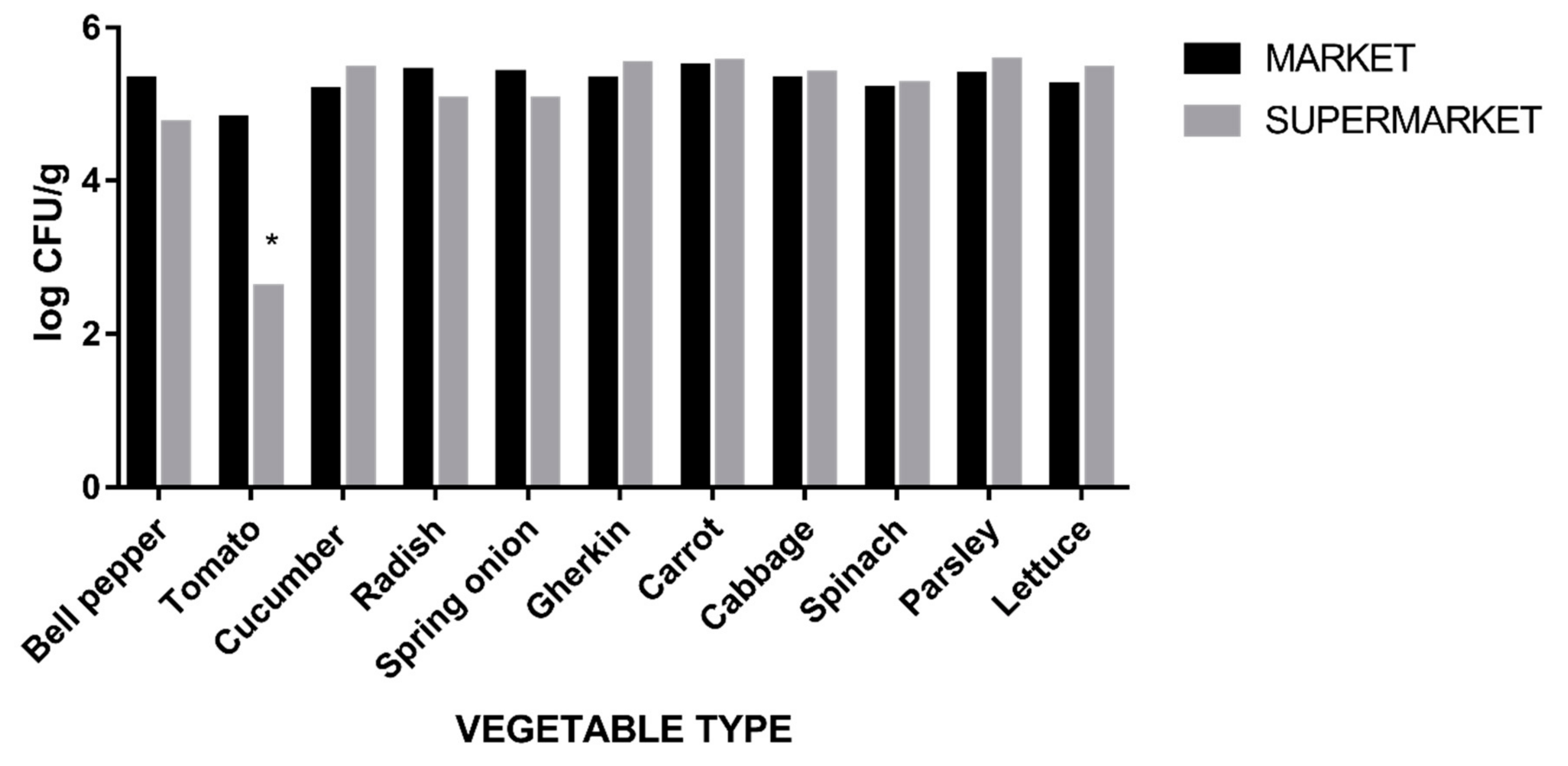
| Vegetable Type (n = 165) | Average log10 CFU/g Count (95% CI) |
|---|---|
| Bell pepper | 5.36 (4–5.77) |
| Tomato | 4.51 (2.6–5.51) |
| Cucumber | 5.34 (5.17–5.49) |
| Radish | 5.22 (4.52–5.48) |
| Spring onion | 5.26 (4.9–5.53) |
| Gherkin | 5,42 (5.1–5.5) |
| Carrot | 5.51 (5.2–5.62) |
| Cabbage | 5.36 (5–5.46) |
| Spinach | 5.23 (4.9–5.32) |
| Parsley | 5.48 (5.14–5.6) |
| Lettuce | 5.36 (5.05–5.44) |
| Bacterial Species | Vegetable Sample | Store Type | Antibiotic Resistance Phenotype | Genetic Determinants |
|---|---|---|---|---|
| Citrobacter brakii | Carrot | Farmer market | AM, AMC, NA, CX, CTX | ND |
| Enterobacter cloacae | Carrot | Farmer market | AM, AMC, CX, CTR | ND |
| Citrobacter freundii | Carrot | Supermarket | AM, AMC, CAZ, CTR, CXM, MEM, IMI, GE, AK, CX, CTX | ND |
| *Serratia marcescens | Spinach | Farmer market | AM, AMC, CAZ, AK, NA, FEP, CTX | TEM, SHV |
| *Morganella morganii | Spinach | Farmer market | AM, AMC, CAZ, CXM, MEM, IMI, GE, AK, NA, CX, CTX | KPC |
| Enterobacter cloacae | Spinach | Supermarket | AM, AMC, CAZ, CTR, CXM, FEP, AK | CTX-M |
| *Escherichia coli | Cucumber | Farmer market | AM, AMC, CAZ, CXM, GE, AK, FEP, DO | CTX-M, TEM |
| Enterobacter cloacae | Cucumber | Supermarket | AM, AMC, CXM, CX | DHA |
| *Enterobacter cloacae | Parsley | Farmer market | AM, AMC, CAZ, CXM, MEM, IMI, GE, AK, NA, CX, FEP, CTX | OXA-48 |
| *Klebsiella oxytoca | Parsley | Supermarket | AM, AMC, CAZ, CTR, CXM, MEM, IMI, GE, AK, NA, CX, FEP, CTX | KPC, SHV |
| Proteus vulgaris | Lettuce | Farmer market | AM, AMC, CAZ, CTR, CXM, FEP, AK | TEM, SHV |
| Enterobacter ludwigii | Lettuce | Farmer market | AM, AMC, CX, CTX | ND |
| *Enteroacter cloacae | Cabbage | Supermarket | AM, AMC, CXM, GE, COT, FEP, CTX | CTX-M, SHV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosi, I.A.; Baciu, A.M.; Opriș, R.V.; Peca, L.; Gudat, T.; Simon, L.M.; Colosi, H.A.; Costache, C. Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania. Foods 2020, 9, 1726. https://doi.org/10.3390/foods9121726
Colosi IA, Baciu AM, Opriș RV, Peca L, Gudat T, Simon LM, Colosi HA, Costache C. Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania. Foods. 2020; 9(12):1726. https://doi.org/10.3390/foods9121726
Chicago/Turabian StyleColosi, Ioana Alina, Alina Mihaela Baciu, Răzvan Vlad Opriș, Loredana Peca, Tristan Gudat, Laura Mihaela Simon, Horațiu Alexandru Colosi, and Carmen Costache. 2020. "Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania" Foods 9, no. 12: 1726. https://doi.org/10.3390/foods9121726
APA StyleColosi, I. A., Baciu, A. M., Opriș, R. V., Peca, L., Gudat, T., Simon, L. M., Colosi, H. A., & Costache, C. (2020). Prevalence of ESBL, AmpC and Carbapenemase-Producing Enterobacterales Isolated from Raw Vegetables Retailed in Romania. Foods, 9(12), 1726. https://doi.org/10.3390/foods9121726







