A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Characterization
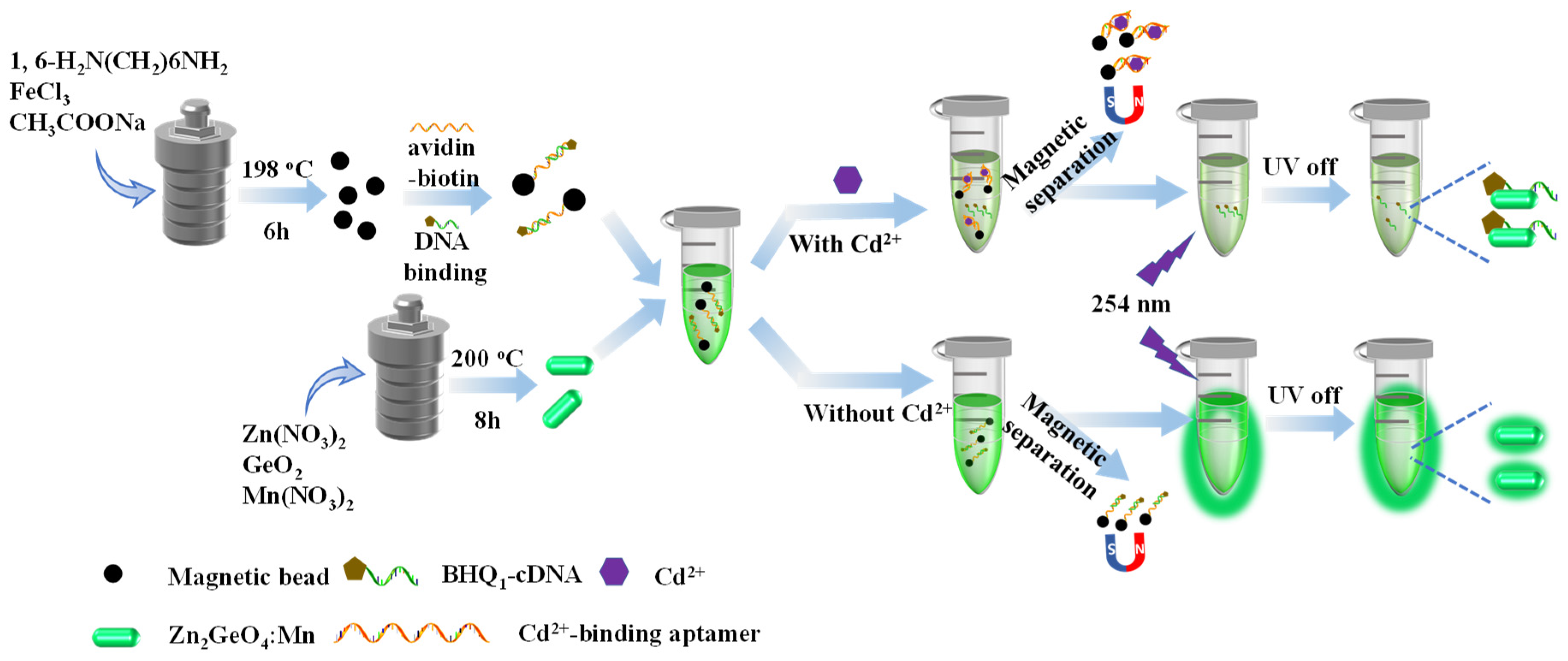
2.3. Synthesis of Phosphorescence Nanorods
2.4. Synthesis of Amine-Functionalized Magnetic Beads
2.5. Immobilization of Cd2+-Binding Aptamers onto Magnetic Beads and Complementary DNA Hybridization
2.6. Detection of Cd2+
2.7. Selectivity of Cd2+
2.8. Food Sample Preparation
2.9. Statistical Analysis
3. Results and Discussion
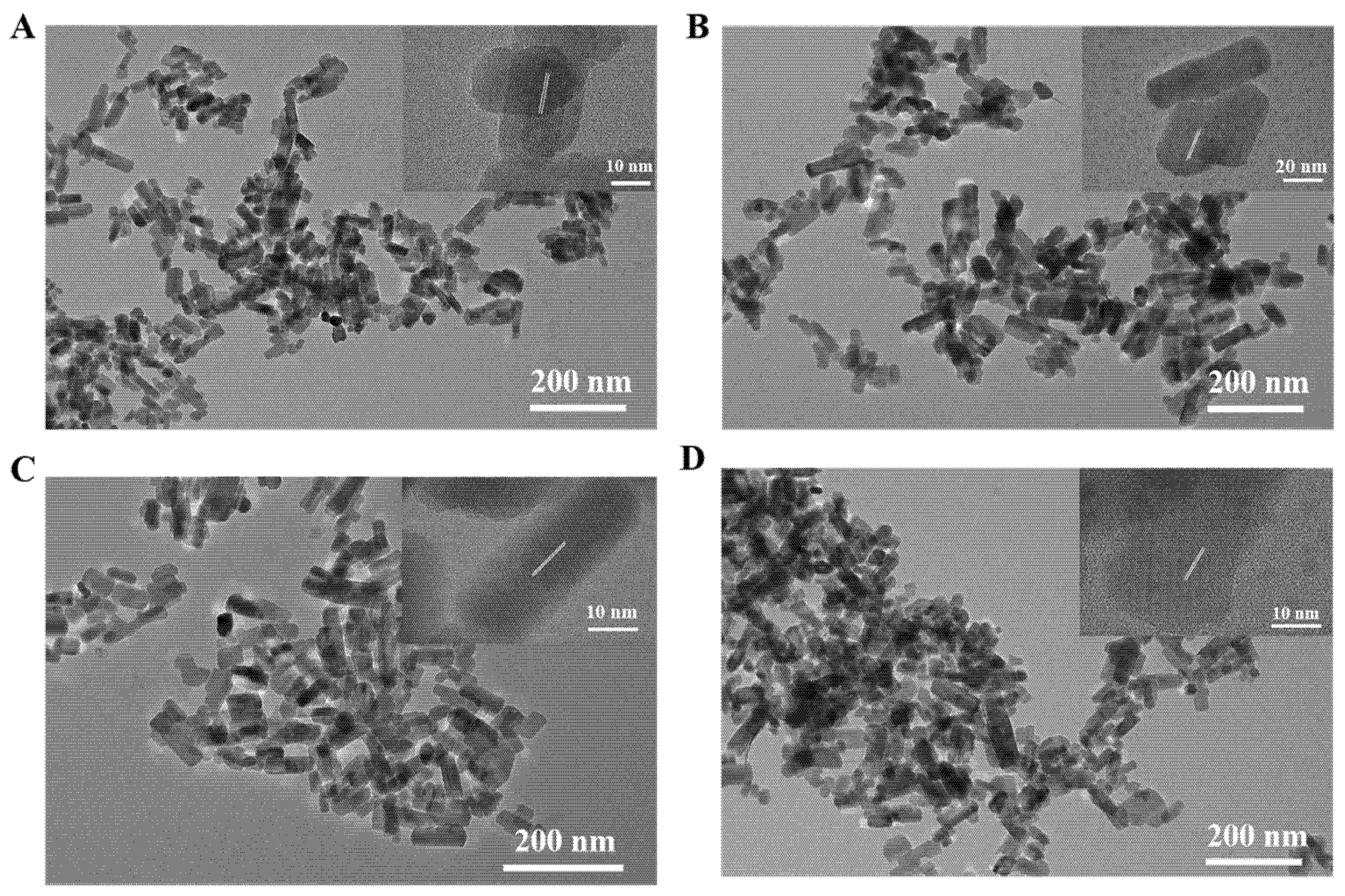
3.1. Characterization of Zn2GeO4:Mn Nanorods
3.2. Characterization of Magnetic Probes for Cd2+ Detection
3.3. Optimization of Experimental Conditions for the Cd2+ Detection
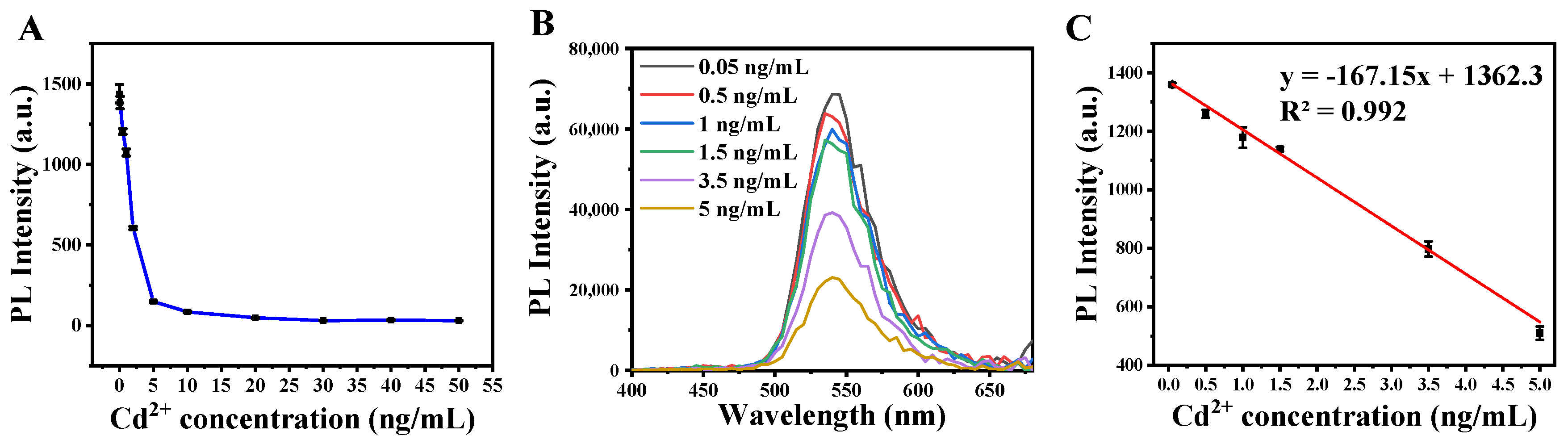
3.4. Quantification of the Cd2+ Assay
3.5. Selectivity Test and Cd2+ Assay in Real Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moulis, J.-M.; Thevenod, F. New perspectives in cadmium toxicity: An introduction. Biometals 2010, 23, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, Y.; Long, M.F.; Luo, T.W.; Bian, J.C.; Liu, X.Z.; Gu, J.H.; Zou, H.; Song, R.L.; Wang, Y.; et al. Beclin-1-mediated autophagy protects against cadmium-activated apoptosis via the Fas/FasL pathway in primary rat proximal tubular cell culture. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Wei, L.; Wu, S.; Duan, N.; Li, X.; Wang, Z. A colorimetric aptamer-based method for detection of cadmium using the enhanced peroxidase-like activity of Au-MoS2 nanocomposites. Anal. Biochem. 2020, 608, 113844. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gou, H.L.; Al-Ogaidi, I.; Wu, N.Q. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.Q.; Hu, X.X.; Liu, H.Y.; Zheng, W.; Chen, X.Y.; Yuan, Q.; Tan, W.H. Autofluorescence-free targeted tumor imaging based on luminous nanoparticles with composition-dependent size and persistent luminescence. ACS Nano 2017, 11, 8010–8017. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed strategies for fluorescence-based biosensors for the detection of mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef]
- Wang, G.K.; Lu, Y.F.; Yan, C.L.; Lu, Y. DNA-functionalization gold nanoparticles based fluorescence sensor for sensitive detection of Hg2+ in aqueous solution. Sens. Actuator B-Chem. 2015, 211, 1–6. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Xing, F.F.; He, S.J.; Song, S.P.; Wang, L.H.; Long, Y.T.; Li, D.; Fan, C.H. A graphene-based fluorescent nanoprobe for silver(I) ions detection by using graphene oxide and a silver-specific oligonucleotide. Chem. Commun. 2010, 46, 2596–2598. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Chen, J.R.; Peng, H. A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens. Bioelectron. 2015, 68, 225–231. [Google Scholar] [CrossRef]
- Zuou, B.; Chen, Y.T.; Yang, X.Y.; Wang, Y.S.; Hu, X.J.; Suo, Q.L. An ultrasensitive colorimetric strategy for detection of cadmium based on the peroxidase-like activity of g-quadruplex-Cd(II) specific aptamer. Anal. Sci. 2019, 35, 277–282. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Wang, Y.-S.; Zhou, B.; Yu, J.-H.; Peng, L.-L.; Huang, Y.-Q.; Li, X.-J.; Chen, S.-H.; Tang, X.; Wang, X.-F. A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium(II). Anal. Bioanal. Chem. 2017, 409, 4951–4958. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mou, Q.B.; Wang, D.L.; Zhu, X.Y.; Yan, D.Y. Dendritic polymers for theranostics. Theranostics 2016, 6, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.S.; Wark, A.W.; Birch, D.J.S.; Gregory, C.D. Phenotypic analysis of extracellular vesicles: A review on the applications of fluorescence. J. Extracell. Vesicles 2020, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Q.Q.; Wang, Y.Q.; Shen, H.J.; Yuan, Q. Recent progress in biomedical applications of persistent luminescence nanoparticles. Nanoscale 2017, 9, 6204–6218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Q.Q.; Zheng, W.; Liu, H.Y.; Yin, C.Q.; Wang, F.B.; Chen, X.Y.; Yuan, Q.; Tan, W.H. One-dimensional luminous nanorods featuring tunable persistent luminescence for autofluorescence-free biosensing. ACS Nano 2017, 11, 8185–8191. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.X.; Shao, J.J.; Jing, X.H.; Zheng, W.W.; Liu, H.; Zhao, Y. Autoluminescence-free dual tumor marker biosensing by persistent luminescence nanostructures. ACS Sustain. Chem. Eng. 2020, 8, 686–694. [Google Scholar] [CrossRef]
- Wu, Y.G.; Zhan, S.S.; Wang, L.M.; Zhou, P. Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Gupta, S.K.; Li, Y.; Mao, Y.B. Bright persistent green emitting water-dispersible Zn2GeO4:Mn nanorods. Dalton Trans. 2020, 49, 7328–7340. [Google Scholar] [CrossRef]
- Lai, B.; Cui, G.; Wang, H.; Song, Y.; Tan, M. Identification of fluorescent nanoparticles from roasted sweet potato (Ipomoea batatas) during normal cooking procedures. LWT 2020, 134, 109989. [Google Scholar] [CrossRef]
- Wang, L.Y.; Bao, J.; Wang, L.; Zhang, F.; Li, Y.D. One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chem. Eur. J. 2006, 12, 6341–6347. [Google Scholar] [CrossRef]
- Ning, H.; Xie, H.; Zhao, Q.S.; Liu, J.L.; Tian, W.; Wang, Y.X.; Wu, M.B. Electrospinning ZnO/carbon nanofiber as binder-free and selfsupported anode for Li-ion batteries. J. Alloys Compd. 2017, 722, 716–720. [Google Scholar] [CrossRef]
- Kogularasu, S.; Akilarasan, M.; Chen, S.M.; Elaiyappillai, E.; Johnson, P.M.; Chen, T.W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. A comparative study on conventionally prepared MnFe2O4 nanospheres and template-synthesized novel MnFe2O4 nano-agglomerates as the electrodes for biosensing of mercury contaminations and supercapacitor applications. Electrochim. Acta 2018, 290, 533–543. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, Y.Y.; Zhu, X.Y.; Zhou, H.C.; Yao, Y.; Li, X.D. Mn2+-doped Zn2GeO4 for photocatalysis hydrogen generation. Int. J. Energy Res. 2019, 43, 5013–5019. [Google Scholar] [CrossRef]
- Bruce, I.J.; Taylor, J.; Todd, M.; Davies, M.J.; Borioni, E.; Sangregorio, C.; Sen, T. Synthesis, characterisation and application of silica-magnetite nanocomposites. J. Magn. Magn. Mater. 2004, 284, 145–160. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Tan, M. Endogenous fluorescence carbon dots derived from food items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef]
- Wei, H.; Li, B.L.; Li, J.; Wang, E.K.; Dong, S.J. Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem. Commun. 2007, 3735–3737. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Teodoro, K.B.R.; Correa, D.S. Green-synthesized gold nanoparticles supported oncellulose nanowhiskers for easy-to-interpret colorimetric detection of cadmium(II). Cell. Chem. Technol. 2020, 54, 407–413. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, T.; Hu, Q.; Zhong, L.; Wang, X.; Wan, H.; Wang, P. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system. Talanta 2020, 208. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, X.-Y.; Wang, Y.-S.; Yi, J.-C.; Zeng, Z.; Zhang, H.; Chen, Y.-T.; Hu, X.-J.; Suo, Q.-L. Label-free fluorescent aptasensor of Cd2+ detection based on the conformational switching of aptamer probe and SYBR green I. Microchem. J. 2019, 144, 377–382. [Google Scholar] [CrossRef]




| Sample | Added | Detected (Mean n = 3) | Recovery (%) |
|---|---|---|---|
| Spring water | 1 ng mL−1 | 1.08 ± 0.08 ng mL−1 | 108.46 ± 8.04 |
| 3 ng mL−1 | 3.36 ± 0.03 ng mL−1 | 112.00 ± 0.98 | |
| 5 ng mL−1 | 5.45± 0.16 ng mL−1 | 109.00 ± 3.16 | |
| surf clam | 0.025 mg kg−1 | 0.027 ± 0.002 mg kg−1 | 108.00 ± 0.08 |
| 0.087 mg kg−1 | 0.089 ± 0.002 mg kg−1 | 101.41 ± 2.58 | |
| 0.125 mg kg−1 | 0.135 ± 0.002 mg kg−1 | 108.05 ± 1.22 |
| Methods | Materials | LOD | Real Samples | Reference |
|---|---|---|---|---|
| Colorimetric | Cellulose nano-whiskers, AuNPs | 60 nM | Water | [27] |
| Colorimetric | Aptamer, AuNPs | 14.56 nM | Water | [28] |
| Colorimetric | Aptamer, AuNP, MoS2 | 9.1 nM | White wine | [3] |
| Fluorescence | Aptamer, SYBR® green Ι | 4.42 nM | Water | [29] |
| Fluorescence | Aptamer, FAM | 2.15 nM | Water | [11] |
| Phosphorescence | Aptamer, Zn2GeO4:Mn, BHQ1 | 0.52 nM | Water, Clams | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, B.; Wang, R.; Yu, X.; Wang, H.; Wang, Z.; Tan, M. A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples. Foods 2020, 9, 1758. https://doi.org/10.3390/foods9121758
Lai B, Wang R, Yu X, Wang H, Wang Z, Tan M. A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples. Foods. 2020; 9(12):1758. https://doi.org/10.3390/foods9121758
Chicago/Turabian StyleLai, Bin, Ruiying Wang, Xiaoting Yu, Haitao Wang, Zhouping Wang, and Mingqian Tan. 2020. "A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples" Foods 9, no. 12: 1758. https://doi.org/10.3390/foods9121758
APA StyleLai, B., Wang, R., Yu, X., Wang, H., Wang, Z., & Tan, M. (2020). A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples. Foods, 9(12), 1758. https://doi.org/10.3390/foods9121758






