Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carrot Juice Preparation
2.3. Preparation of Starter Cultures
2.4. Carrot Juice Fermentation Experiments
2.5. Microbial Analysis
2.6. Analysis of Sugars by HPLC
2.7. Determination of pH and Titratable Acidity
2.8. Extraction and Assay of Fructosyltransferase Enzymes
2.9. Fourier Transform Infrared (FTIR) and Raman Spectroscopy Analysis
2.10. Data Analysis
3. Results and Discussion
3.1. Production of Fructosyltransferase Enzymes in Fermented Carrot Puree
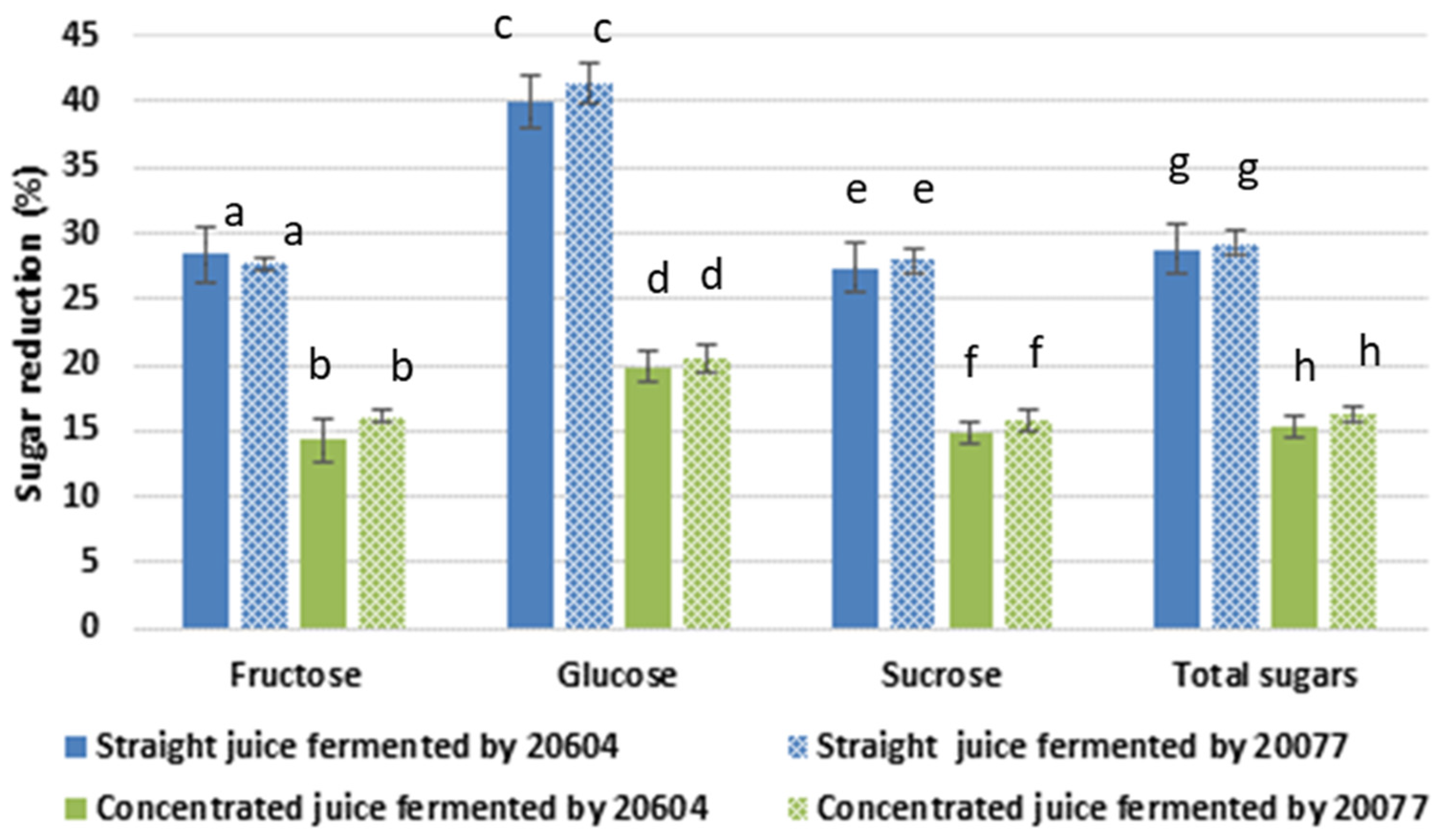
3.2. Sugar Reduction and Change in Total Polysaccharide Content
3.3. Change in Titratable Acidity of Carrot during Fermentation
3.4. Polysaccharide, Carotenoid, and Carotene Content Analysed from Raman and FTIR Intensity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Terefe, N.S.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2019, 60, 2887–2913. [Google Scholar] [CrossRef]
- Bergqvist, S.W.; Andlid, T.; Sandberg, A.-S. Lactic acid fermentation stimulated iron absorption by Caco-2 cells is associated with increased soluble iron content in carrot juice. Br. J. Nutr. 2006, 96, 705–711. [Google Scholar]
- Rakin, M.; Baras, J.; Vukasinovic, M.; Maksimovic, M. The examination of parameters for lactic acid fermentation and nutritive value of fermented juice of beetroot, carrot and brewer’s yeast autolysate. J. Serbian Chem. Soc. 2004, 69, 625–634. [Google Scholar] [CrossRef]
- Ye, J.-H.; Huang, L.-Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus amyloliquefaciens JN4 acts as a prebiotic for enhancing the intestinal adhesion capacity of Lactobacillus reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; Rivas, B.D.L.; De Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Cai, Y.X.; Wang, J.H.; McAuley, C.; Augustin, M.A.; Cai, Y. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite profile changes and increased antioxidative and antiinflammatory activities of mixed vegetables after fermentation by Lactobacillus plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y.J.; Kim, Y.; Kim, S. A synbiotic combination of Lactobacillus gasseri 505 and Cudrania tricuspidata leaf extract prevents hepatic toxicity induced by colorectal cancer in mice. J. Dairy Sci. 2020, 103, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Shirouchi, B.; Nagao, K.; Umegatani, M.; Shiraishi, A.; Morita, Y.; Kai, S.; Yanagita, T.; Ogawa, A.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 improves glucose tolerance and reduces body weight gain in rats by stimulating energy expenditure. Br. J. Nutr. 2016, 116, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzmeier, T.D.; Mudaliar, N.S.; Stanbro, J.A.; Watters, C.; Ahmad, A.; Simons, M.P.; Ventolini, G.; Zak, J.C.; Colmer-Hamood, J.A.; Hamood, A.N. Application of Lactobacillus gasseri 63 AM supernatant to Pseudomonas aeruginosa-infected wounds prevents sepsis in murine models of thermal injury and dorsal excision. J. Med. Microbiol. 2019, 68, 1560–1572. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Luongo, D.; Sashihara, T.; Rossi, B.M.; Siciliano, R.A. Secretome Analysis of Mouse Dendritic Cells Interacting with a Probiotic Strain of Lactobacillus gasseri. Nutrients 2020, 12, 555. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Zhu, Y.; Xu, W.; Bai, Y.; Zhang, T.; Mu, W. Biosynthesis of inulin from sucrose using inulosucrase from Lactobacillus gasseri DSM 20604. Int. J. Biol. Macromol. 2018, 109, 1209–1218. [Google Scholar] [CrossRef]
- Díez-Municio, M.; Herrero, M.; Rivas, B.D.L.; Muñoz, R.; Jimeno, L.; Moreno, F.J. Synthesis and structural characterization of raffinosyl-oligofructosides upon transfructosylation by Lactobacillus gasseri DSM 20604 inulosucrase. Appl. Microbiol. Biotechnol. 2016, 100, 6251–6263. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Kralj, S.; Piqué, A.V.; Leemhuis, H.; Van Der Maarel, M.J.E.C.; Dijkhuizen, L. Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: Characterization of three novel fructansucrase enzymes and their fructan products. Microbiol. 2010, 156, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [Green Version]
- Kilian, S.; Kritzinger, S.; Rycroft, C.; Gibson, G.; Du Preez, J. The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonic microbiota. World J. Microbiol. Biotechnol. 2002, 18, 637–644. [Google Scholar] [CrossRef]
- Liu, C.; Kolida, S.; Charalampopoulos, D.; Rastall, R.A. An evaluation of the prebiotic potential of microbial levans from Erwinia sp. 10119. J. Funct. Foods 2020, 64, 103668. [Google Scholar] [CrossRef]
- Díez-Municio, M.; Rivas, B.D.L.; Jimeno, M.L.; Muñoz, R.; Moreno, F.J.; Herrero, M. Enzymatic Synthesis and Characterization of Fructooligosaccharides and Novel Maltosylfructosides by Inulosucrase from Lactobacillus gasseri DSM 20604. Appl. Environ. Microbiol. 2013, 79, 4129–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koley, T.K.; Nishad, J.; Kaur, C.; Su, Y.; Sethi, S.; Saha, S.; Sen, S.; Bhatt, B.P. Effect of high-pressure microfluidization on nutritional quality of carrot (Daucus carota L.) juice. J. Food Sci. Technol. 2020, 57, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.; Balaban, M.; MAlTHEWS, R. Optimization of Carrot Juice Color and Cloud Stability. J. Food Sci. 1993, 58, 1129–1131. [Google Scholar] [CrossRef]
- Da Silva, I.M.; Rabelo, M.C.; Rodrigues, S. Cashew juice containing prebiotic oligosaccharides. J. Food Sci. Technol. 2012, 51, 2078–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Chen, Y.; Wang, X. Extraction and deproteinization of pumpkin polysaccharide. Int. J. Food Sci. Nutr. 2011, 62, 568–571. [Google Scholar] [CrossRef]
- Nawaz, H.; Bonnier, F.; Knief, P.; Howe, O.; Lyng, F.M.; Meade, A.D.; Byrne, H.J. Evaluation of the potential of Raman microspectroscopy for prediction of chemotherapeutic response to cisplatin in lung adenocarcinoma. Analyst 2010, 135, 3070–3076. [Google Scholar] [CrossRef] [Green Version]
- Cadusch, P.J.; Hlaing, M.M.; Wade, S.A.; McArthur, S.L.; Stoddart, P.R. Improved methods for fluorescence background subtraction from Raman spectra. J. Raman Spectrosc. 2013, 44, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Hlaing, M.M.; Dunn, M.; Stoddart, P.R.; McArthur, S.L. Raman spectroscopic identification of single bacterial cells at different stages of their lifecycle. Vib. Spectrosc. 2016, 86, 81–89. [Google Scholar] [CrossRef]
- Sakamoto, H. Changes in Carrot Juice Components Bacteria Food Science and Technology International. Food Bioprocess Technol. 1996, 2, 246–252. [Google Scholar]
- Hu, R.-K.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef]
- Malik, M.; Bora, J.; Sharma, V. Growth studies of potentially probiotic lactic acid bacteria (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus casei) in carrot and beetroot juice substrates. J. Food Process. Preserv. 2019, 43, 8. [Google Scholar] [CrossRef]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near infrared Raman spectra of human brain lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.; Wagner, M.; Horn, H.; Niessner, R.; Haisch, C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal. Bioanal. Chem. 2009, 393, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Kacurã¡kovã¡, M. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Demir, N.; Acar, J. Effects of storage on quality of carrot juices produced with lactofermentation and acidification. Eur. Food Res. Technol. 2004, 218, 465–468. [Google Scholar] [CrossRef]
- Chavasit, V.; Pisaphab, R.; Sungpuag, P.; Jittinandana, S.; Wasantwisut, E. Changes in β-Carotene and Vitamin A Contents of Vitamin A-rich Foods in Thailand During Preservation and Storage. J. Food Sci. 2002, 67, 375–379. [Google Scholar] [CrossRef]
- Hagi, T.; Kobayashi, M.; Nomura, M. Aerobic conditions increase isoprenoid biosynthesis pathway gene expression levels for carotenoid production in Enterococcus gilvus. FEMS Microbiol. Lett. 2015, 362, fnv075. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E. Lactic Acid Fermentation of Tomato: Effects on cis/trans Lycopene Isomer Ratio, beta-Carotene Mass Fraction and Formation of L(+)- and D(-)-Lactic Acid. Food Technol. Biotechnol. 2013, 51, 471–478. [Google Scholar]




| Sample | Enzyme Activity (U/L Juice) | Relative Change in Total Polysaccharide Content | ||
|---|---|---|---|---|
| Total Activity | Hydrolytic Activity | Transglycosylation Activity | ||
| Fermented straight DSM 20604 | 570 ± 115.6 a | 290 ± 59.3 b | 280.9 | 1.77 ± 0.17 c |
| Fermented straight DSM 20077 | 530 ± 51.9 a | 290 ± 32.3 b | 240.7 | 1.25 ± 0.22 c |
| Fermented concentrated DSM 20604 | 370 ± 171.5 a | 250 ± 10.6 b | 113.5 | 1.64 ± 0.04 c |
| Fermented concentrated DSM 20077 | 350 ± 44.2 a | 290 ± 62.1 b | 61.2 | 1.51 ± 0.27 c |
| Wave Number (cm−1) | Peak Assignment |
|---|---|
| FTIR spectra | |
| 963–1018 | υ(CO), υ(CC), def(OCH), ring (polysaccharides, pectin) |
| 1020–1050 | Glycogen absorption due to str(C-O and C-C) and def(C-O-H) |
| 1400–1500 | Symmetric CH3 bending of the methyl groups of proteins, str(C-N), def(N-H), def(C-H) |
| 1480–1543 | Amide II |
| Raman spectra | |
| 954–956 | Carotenoids |
| 1008 | υ(CO), υ(CC), def(OCH), ring (polysaccharides, pectin) |
| 1150–1157 | Carotenoid peaks due to C-C and conjugated C=C band stretch, C-C, C-N stretching (protein), |
| 1313–1314 | CH3CH2 twisting mode of collagen/lipid |
| 1325–1339 | CH3CH2 wagging mode in purine bases of nucleic acids and tryptophan |
| 1370 | The most pronounced saccharide band |
| 1437–1453 | defCH2 |
| 1462 | defCH2 of disaccharides, sucrose |
| 1491 | C-N stretching vibration coupled with the in-plane C-H bending in amino radical cations |
| 1517–1520 | β-carotene accumulation (C-C stretch mode) |
| 1520–1538 | Carotenoid peaks due to C-C and conjugated C=C band stretch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Hlaing, M.M.; Glagovskaia, O.; Augustin, M.A.; Terefe, N.S. Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice. Foods 2020, 9, 1803. https://doi.org/10.3390/foods9121803
Xu Y, Hlaing MM, Glagovskaia O, Augustin MA, Terefe NS. Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice. Foods. 2020; 9(12):1803. https://doi.org/10.3390/foods9121803
Chicago/Turabian StyleXu, Yue, Mya Myintzu Hlaing, Olga Glagovskaia, Mary Ann Augustin, and Netsanet Shiferaw Terefe. 2020. "Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice" Foods 9, no. 12: 1803. https://doi.org/10.3390/foods9121803
APA StyleXu, Y., Hlaing, M. M., Glagovskaia, O., Augustin, M. A., & Terefe, N. S. (2020). Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice. Foods, 9(12), 1803. https://doi.org/10.3390/foods9121803




