3.2. Analysis of the Gelation Process Using Rheology
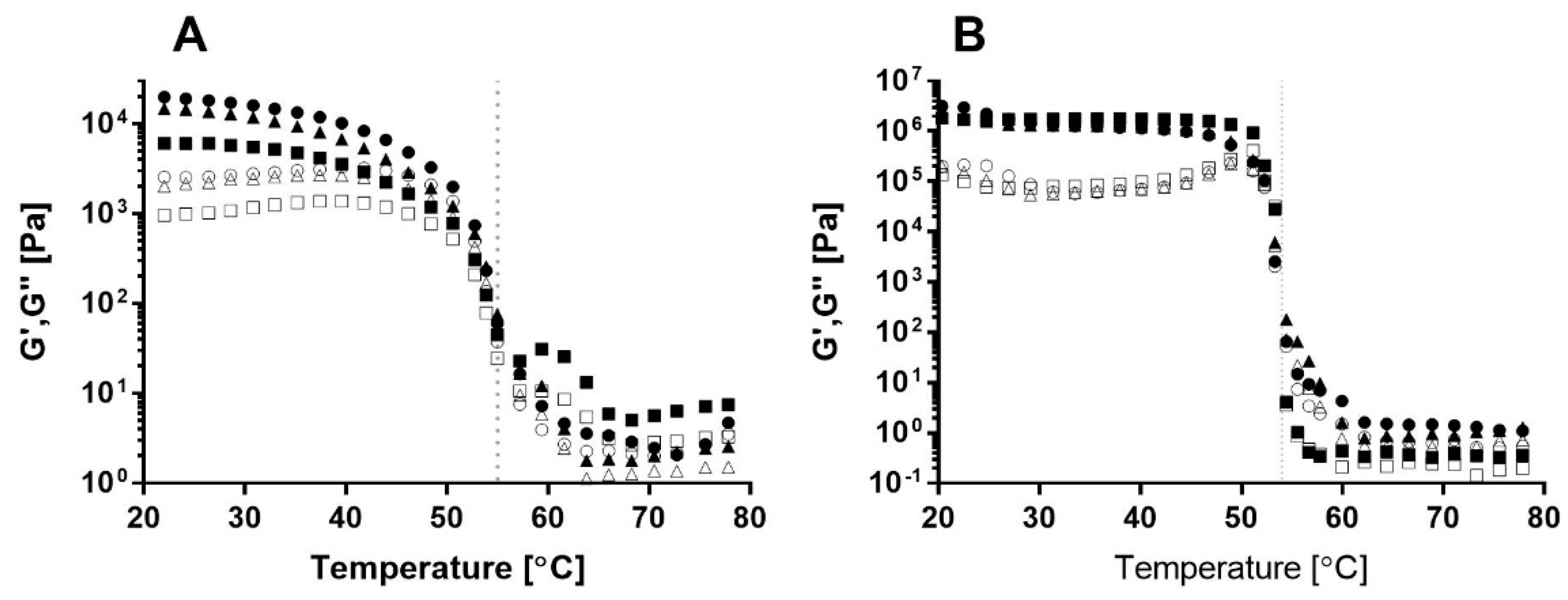
The gelation process of the different bigels with different SEs was monitored by temperature-dependent dynamic oscillatory rheological measurements (80 °C–20 °C, see
Figure 1A). In this analysis, the viscoelastic properties of the sample were expressed by (a) the storage modulus, G’, which is defined as the elastic characteristic of the sample and represents the solid-like behavior of the material, and (b) the loss modulus, G’’, which is defined as the viscous characteristic of the material and represents the flow and mobility of the sample [
28]. A clear transition from viscous liquid-like behavior, characterized by G’’~G’, to solid-like behavior, characterized by G’>G’’, is evident during the cooling process of the samples [
29]. The sol–gel transition temperature or characteristic gelation temperature was recorded as the temperature at which G’ rapidly increased during the cooling process and a significant difference between the G’’ and G’ was detected, with G’>>G’’ [
30].
A gelation temperature of approximately 55 °C was recorded for all bigel formulations and was reflected by a sharp increase in both moduli during cooling (
Figure 1A). GMS crystallization in oil occurred around 40–60 °C, depending on oil type, GMS concentration, and gelation conditions [
20,
21,
31], and gelatin gelation in water occurred around 18–35 °C, depending on gelatin concentration and setting temperature [
32]. Moreover, a previous study reported lower gelation temperatures in the range of 25–55 °C for GMS oleogels prepared using various concentrations in the range between 0.75 and 8 wt % [
33]. These results suggest that the sol–gel transition detected in the current analysis is governed by GMS crystallization rather than by gelatin gelation.
Similar gelation temperatures were observed for the different samples with various SEs, suggesting that the emulsifier’s HLB value did not affect the gelation process, as expressed by the gelation temperature. Bin Sintang et al. [
34] studied the gelation of oil using combinations of SEs and lecithin and found that a decrease in the SE:lecithin ratio shifted the gelation transition of the oleogel to a lower temperature. This behavior was attributed to interactions between SE and lecithin and their organization in the oil phase. To evaluate the involvement of SE in oleogel stabilization and its effect on bigel gelation, the oil phase content, i.e., the oleogel, was examined using the same procedure (
Figure 1B). As can be seen, the sol–gel transition of the oleogels with different SEs occurred at the same temperature, suggesting that the HLB value does not affect the gelation temperature of the oil phase. Moreover, the gelation temperatures of the oleogel and bigel are similar, supporting our assumption that bigel gelation is governed by GMS crystallization, leading to oil phase stabilization. It is interesting to note that the gelation transition of the oil phase (
Figure 1B) appears as a sharp, distinct transition, while the gelation transition of the bigel (
Figure 1A) is more moderate. This can be attributed to the presence of a water phase in the bigel that might interfere with the gelation process.
Thus, it can be concluded that the bigel gelation detected in the temperature sweep analysis is governed by the gelation of the oil phase, i.e., GMS, and that it is unaffected by the HLB value of the SE.
3.3. Thermal Analysis
The gelation thermal transition was also monitored using DSC in order to verify the involvement of the GMS crystallization in the bigel gelation as suggested in the temperature-dependent rheology analysis. More specifically, the melting temperaure of the oil phase and the evaporation temperature of the water phase were determined using DSC.
The heat flow required to heat the samples using a 3 °C min
−1 heating rate demonstrated three main endothermal peaks (
Figure 2). The first peak, at ≈59 °C, can be related to the melting temperature of GMS in oil [
35]. This peak was observed only in the bigel thermograms and was absent from the water phase thermogram, supporting our assumption. No difference was found in the location of the first peak in the thermograms of the different bigels, suggesting that the esterification degree of the SE had no effect on the melting temperature of GMS crystals in the oil phase. This observation is in line with the results of the temperature sweep analysis, suggesting that the SE type does not affect the oil phase sol–gel transition.
Two additional endothermic peaks were identified at ≈94 and ≈103 °C, respectively. The melting temperature of gelatin hydrogels ranges between 10 and 40 °C [
18], while the evaporation temperature of glycerol is about 290 °C [
36]. Therefore, these peaks were attributed to the evaporation of water from the bigel system. The water dehydration process can be divided into two steps: dehydration of free water and dehydration of bound water. The differences between these two processes relate to the temperatures at which the water molecules dehydrate, whereby free water dehydrates at a lower temperature compared with bound water molecules [
37]. Therefore, the second and third peaks were associated with the free and bound water within the water phase, respectively. Bellich et al. [
38] characterized the evaporation behavior of aqueous alginate solutions to which different molecules were added. In the study of Bellich et al., a single endothermic peak was detected for the water evaporation process, which was characterized by an exponential increase in heat flux as a function of temperature, ending in a maximum followed by a sharp decrease. The effect of different solutes on the peak maximum temperature and the sharp decrease observed were related to the ability of each solute to bind water. A similar water hydration process, characterized by one endothermic peak with peak broadening due to solute content, was observed in water/poly(vinyl alcohol)/glycerol systems [
39]. In the current research, two separate distinct endothermic peaks were detected for the free and bound water. It seems that the heating rate used in the current research, i.e., 3 °C min
−1, as opposed to the heating rates used in the above studies, i.e., 5 and 10 °C min
−1, yielded better separation between the peaks of the bound and unbound water.
The presence of two distinct peaks reflecting water evaporation was seen in all samples, i.e., water phase containing gelatin, glycerol, and water, and different bigels. However, the bound water peak, i.e., the third, high temperature peak, was broader and smaller in the bigels compared with the water phase samples. This phenomenon can be attributed to gelatin diffusion to the water/oil interface during emulsification, which acts as a long-term stabilizer of the emulsion [
13]. The water phase analysis (without the presence of the oil phase) examines hydrated gelatin molecules in the water phase, and so maximum water molecules are bound to the polymer backbone. The results in
Figure 2 suggest that combining oil and water phases, such as in the case of the examined bigels, leads to a decrease in the amount of bound water, which can be the result of a decrease in the amount of fully hydrated gelatin molecules. Such behavior can be related to the diffusion of gelatin molecules to the water/oil interface, decreasing the amount of hydrated gelatin molecules in the water. Thus, less water molecules were bound to the gelatin and the magnitude of the third peak decreased.
To further examine the hypothesis that the decrease in the third peak seen in the DSC thermograms (
Figure 2) resulted from a decrease in the amount of bound water in the bigel compared with the water phase, TGA was performed. The bigels and water phase, consisting of glycerol and gelatin, were heated while being weighed to estimate the amount of bound and free water in each sample. Typical weight loss and derivative of weight loss thermograms are presented in
Figure 3A,B, respectively.
Figure 3A presents two main weight loss regions, the first around 90 °C and the second around 360 °C, as well as a smaller weight loss region around 300 °C. The typical boiling temperature of vegetable oils is around 390 °C [
40], and so the higher temperature weight loss region (≈360 °C) was attributed to the evaporation and decomposition of the oil phase. The smaller weight loss region around 300 °C was attributed to the decomposition of gelatin and glycerol, as determined by TGA measurements done on the pure components. The decomposition of proteins [
41] in general, and specifically of gelatin and glycerol [
42], were previously detected at this temperature range. Finally, the first weight loss region, around 90 °C, can be related to water evaporation [
38]. This water dehydration step was found to be ≈40 wt % out of the bigel samples (
Figure 3A), which is close to the percent of water used to prepare the bigels, i.e., 47% (
Table 1). Moreover, the main weight loss of the water phase consisting of gelatin, glycerol, and water (gray line) occurred in this temperature range, supporting our assumption that this region in the bigel thermograms is related to water dehydration. Thus, to examine the behavior of the bound and free water in the bigel samples, the analysis concentrated on this region.
Figure 3B presents the derivative of the percentage of weight loss (derivative thermogravimetry, DTG) with temperature, as a function of temperature, between 50 and 180 °C, for the different samples. The curve obtained from the water phase (gray line) has a single peak with a maximum at ≈135 °C. The onset temperature of this peak is around 30 °C, and the evaporation curve increases gradually from this temperature until it reaches a maximum followed by a sharp drop. This behavior is characteristic of the water evaporation process seen in TGA [
38,
39]. The location of this peak maximum at such a high temperature suggests that the water molecules in the water phase are bound, as suggested also by the DSC analysis. The DTG curves for the bigels exhibited two main temperature regions: the first at a lower temperature, ≈86 °C, which can be associated with free water, and the second at a higher temperature, ≈102 °C, which can be associated with bound water. These results are in line with the DSC analysis presented above. It is interesting to note that the magnitude and area of the second peak (≈102 °C) are significantly lower than those of the first peak (≈86 °C), suggesting that the mass fraction of the bound water molecules in the bigel formulation is lower than that of the unbound molecules. Moreover, the presence of two regions in the bigel curves, as opposed to a single region in the water phase curve, suggests that the polymer in the water phase is fully hydrated, while in the bigel, there is a distinct difference between the protein in the bulk water and the protein at the interface. Thus, it seems that part of the water that was bound to the protein in the water phase became unbound after mixing and homogenization with the oil phase. This result supports our assumption that gelatin diffuses to the water–oil interface in bigel systems and thus can potentially affect the emulsification and stability of the bigel system, which will be further discussed below.
3.4. Viscoelastic Properties
The gel’s viscoelastic properties were determined to examine the gelation mechanism and gel type. Frequency sweep tests were applied to various formulations, and the results are presented in
Figure 4. All samples exhibit significantly higher G′ values compared with G′′ over the entire frequency range, suggesting solid-like gel behavior [
29].
Gels can be classified into two main categories: weak physical gels, in which G′ and G′′ depend on the frequency, and strong chemical cross-linked gels, in which G′ and G′′ are relatively frequency-independent [
43]. The results show a slight frequency dependency, as evidenced by the weak positive slope of G′ and G′′ vs. frequency curves. This dependency can be expressed using a power-law model [
44].
where
and
are, respectively, the storage and loss moduli at 1 rad·s
−1, n′ and n′′ are, respectively, the exponent expressing the frequency dependence of G′ and G′′, and
is the angular frequency in s
−1.
Table 2 summarizes the fitted parameters from Equations (4) and (5). In general, all bigel samples exhibited positive frequency-dependent moduli values expressed by an exponent value greater than zero. Such a tendency implies the physical character of the gel, which dictates a change in moduli with frequency [
45]. In general, lower G′ exponent values, n′, were detected compared with the G′′ exponent value, n′′, which is also typical for physical gels [
46]. Exponent values in the order of H1 < H2 < H6 were obtained for both moduli. Higher exponent values imply frequency-dependent behavior, suggesting that the gel network is more sensitive to applied shear. The loss and storage moduli at 1 rad·s
−1, i.e.,
and
, demonstrated opposite trends, with values in the order of H1 > H2 > H6. It is well established that G′ expresses the solid-like behavior of viscoelastic materials, and so a higher G′ value implies a harder gel. Therefore, it can be concluded that H1 and H2 samples exhibited harder mechanical characteristics compared with H6 and that H1 was the hardest bigel while H6 was the softest. A similar trend was seen in the loss and storage modulus at 1 rad·s
−1 of Surimi gels when the polymer concentration was increased, leading to harder gels [
47].
Analysis of the loss and storage moduli ratio, termed the loss factor, i.e., tan δ = G′′/G′, provides an additional point of view on the viscoelastic behavior of the gels. In the frequency range tested, all samples exhibited solid-like characteristics, with tan δ < 1 [
2]. However, a positive frequency-dependent slope was observed, suggesting a moderate softening transition with frequency to a more liquid-like behavior. This trend was more significant for the H6 sample compared with the H2 and H1 samples, as can be seen by the change in the curve slopes (
Figure 4B). The relationship between gel strength and loss factor value was also observed previously by Ojeda-Serna et al. [
15], while analyzing the effect of water content on the viscoelastic properties of W/O myverol oleogel-based emulsion with coconut and canola oils. In this study, higher loss tangent values were obtained when using a lower concentration of water, leading to more solid-like characteristics at lower water concentrations.
In conclusion, bigels produced with SEs with low HLB values exhibit higher elastic properties with more solid-like behavior compared with bigels produced with higher HLB SEs. To further understand the source of this trend, particle size analysis using microscopy was performed.
3.5. Microstructural Analysis
The microstructure of the different bigel formulations obtained using three different types of SEs was studied using confocal microscopy (
Figure 5). This technique was used to determine the location of each phase, i.e., water and oil, and to analyze the droplet size and distribution. In all three formulations, oil droplets (red) were observed within the continuous water phase (blue), verifying the formation of O/W emulsions. Emulsions can be classified according to the relative spatial distribution of the oil and water phases into W/O or O/W emulsion types [
2]. The HLB value of surfactants can provide a useful indication as to which emulsion it will form; low HLB surfactants (3–6) favor the formation of W/O emulsions, while high HLB surfactants (10–18) favor the formation of O/W emulsions. Surfactants with intermediate HLB values (7–9) have no particular preference [
2,
48]. In the current research, we used lecithin, which has an HLB of ≈7, GMS with an HLB around ≈3.8, and three different SEs with HLB values of ≈1, 2, and 6. Based on these HLB numbers, one would expect to obtain W/O emulsions, but it seems that the combination of the different surfactants led to the formation of an O/W type emulsion. Lecithin has a higher HLB value than all of the SEs, and so it was able to stabilize smaller droplets in O/W emulsions [
11,
13]. Moreover, it was shown that a combination of two or more surfactants with different HLB values can be used to create a system with a specific HLB value, thus controlling the formation of a specific emulsion type [
49].
GMS was used in the current system as an oil-structuring agent, although its high concentration and emulsification properties can potentially direct some of the GMS molecules to the water–oil interface. A previous study focused on a combination of lecithin and GMS as emulsifiers in water–oil systems and showed the formation of an O/W type emulsion [
11]. The bigel system also contains gelatin molecules aimed at gelling and stabilizing the water phase, which can also participate in the stabilization of the water–oil interface as discussed in the thermal analysis section (
Section 3.2). The ability of protein to stabilize the water–oil interface is driven by the presence of both hydrophobic and hydrophilic amino acids. This tendency is even stronger when the protein solution temperature is increased, exposing the hydrophobic areas of the protein, which can potentially lead to absorption at the oil droplet interface. The bigel preparation procedure discussed in the current research involved hot emulsification at 70 °C, which can potentially promote protein unfolding and absorption at the water–oil interface due to the high temperature [
50] and the homogenization process [
51]. The ability of gelatin to stabilize O/W emulsions was examined previously; however, relatively low surface activity was suggested for fish gelatin compared with globular proteins such as β-lactoglobulin [
52]. Therefore, it can be concluded that despite the use of surfactants with low or intermediate HLB values, which usually favor the formation of W/O emulsions, the presence of gelatin and GMS led to the formation of O/W emulsions.
The micrographs demonstrated the effect of the different SEs on particle size and distribution, where H1 and H2 formulations demonstrated similar particle size that was relatively smaller compared with the H6 sample. Droplet size and distribution, based on the d
32, d
43, and C
2 values, were determined by analyzing the image of each formulation using ImageJ software (
Table 3). The droplet sizes d
32 and d
43 represent the surface-weighted mean diameter and volume-weighted mean diameter, respectively, while C
2 represents the relative standard deviation. In general, among the common ways to express the mean particle diameter of polydisperse emulsions, it can be said that the higher the order of the mean (a + b sum in d
ab), the higher the numerical value and the more significant the weight of every larger droplet present, increasing the mean [
2]. The results confirm similar d
43 and d
32 values for H1 and H2 samples, which are significantly smaller than the values obtained for the H6 sample. The large differences in mean particle size values may be indicative of large polydispersity, which is demonstrated by the C
2 value. C
2 values range from 0.1 for a very narrow droplet distribution to 1.3 for a very wide distribution [
2]. A narrower particle size distribution will result in comparable d
43 and d
32 values, as can be seen for H1 and H2, while for wide distributions, such as in the case of H6, d
43 values are higher compared with d
32, as are C
2 values. Dickinson and Galazka [
53] examined the stability of n-hexadecane in water emulsions stabilized using β-lactoglobulin by measuring d
43 and d
32 of the emulsions immediately after preparation and after three weeks of storage. They showed that during storage, the difference between d
43 and d
32 values increased significantly due to coalescence, leading to broader distributions.
The difference in droplet size seen in the different samples was attributed to the surfactant located at the O/W interface, which was responsible for emulsion stabilization [
51]. As mentioned above, the emulsion gels studied contained four different surface-active molecules that act as emulsifiers in the system: SE, GMS, lecithin, and gelatin. Due to their different molecular size, these surfactants are expected to have different absorption kinetics with respect to the oil/water interface. In general, during homogenization, surfactants with smaller molecular weights will adsorb on the interface faster than larger molecules and will promote the formation of smaller droplets. This stabilization effect is more pronounced in the short term, while in the long term, the larger molecular architectures will usually provide better stabilization [
13,
54]. In the current research, we focus on the short-term stabilization of small emulsifiers, i.e., SE, lecithin, and GMS, due to the gelation and solidification of the water and oil phases that take place after cooling, which can provide the long-term stabilization effect [
55].
In general, all samples consist of similar amounts of lecithin and GMS, whereas the SE type differed. It is assumed that during emulsification, all surfactants present in the formulation are in liquid state and can “compete” for available space at the oil–water interface based on their molecular size and HLB value. The molecular size of SE, which is directly related to its HLB value, is governed by its esterification degree. The SEs used in the current research are commercially available based on the percent of mono-esterified sucrose molecules present in the sample. As specified by the manufacture, SE SP01 is a fully esterified sample, meaning that all eight positions on the sucrose are esterified with palmitic or stearic acids, SE SP02 contains 10% mono-esterified and 90% fully esterified sucrose molecules, while SP30 has 30% mono-esterified and 70% fully esterified sucrose molecules. This product specification leads to various interfacial coverage abilities, whereby during emulsification, mono-esterified sucrose molecules are able to migrate to the interface faster than fully esterified sucrose. Thus, we expect that the SEs will compete for interface positions according to the following order: SP30>SP02>SP01. Generally, SE molecular weight will vary between ≈580 gmol
−1 for mono-esterified sucrose and ≈2250 gmol
−1 for fully esterified sucrose (based on palmitic acid). On the other hand, the major constituents of soybean lecithin are phosphatidylcholine, phosphatidyl-ethanolamine, and lysophosphatidylcholine, whose molecular weights range between 800 and 900 gmol
−1, depending on the fatty acid bound [
56]. Therefore, it is assumed that in formulation H1, which contains SP01, the lecithin will reach the oil–water interface faster than the SE, due to its smaller size, and so in this sample, less SE will be present on the oil–water interface, and more SE will be found in the oil phase. The opposite is expected when using SP30, which has a higher content of mono-esterified sucrose that can potentially reach the interface fast and compete with the lecithin for available sites on the interface. As a result, the H6 sample is expected to contain more SE and less lecithin at the oil–water interface compared with H1 and H2 samples. Thus, due to the higher HLB value of lecithin compared with the different SEs, and its higher content in the H6 sample, H6 is expected to stabilize smaller droplets in the O/W bigels, as observed in the micrographs and presented in
Table 3. Moreover, the use of SP01 SE by itself, without lecithin, results in unstable emulsions that separate immediately, suggesting that the fully esterified sucrose molecules in SP01 are too big and not amphiphilic enough to stabilize this kind of emulsion gels. It is suspected that due to the high esterification degree of SP01, the steric interference between the molecules is too high, and so, the molecules cannot arrange properly on the interface, leading to de-stabilization. It seems that the SP02 sucrose sample, which contains only 10% mono-esterified sucrose, behaves similar to the fully esterified sample, SP01. Moreover, due to the lower presence of SE in the interface in H1 and H2 bigel systems, more SE is expected to be found in the oil phase compared with the case of H6, thus strengthening the oleogel network within the oil phase. This phenomenon will be further discussed in
Section 3.5, Mechanical Properties.
3.6. Mechanical Properties
The distribution of the oil droplets in the continuous water phase during the hot emulsification preparation procedure is expected to affect the final mechanical properties of the gel due to various effects such as droplet size, distribution, oleogel hardness, and water/oil interactions. The effect of surfactant type on the final mechanical properties of the bigel was investigated using texture profile analysis (TPA) and spreadability tests.
Table 4 presents the textural attributes calculated from the TPA of the different bigels at room temperature. The results demonstrate hardness values in the order of H1 > H2 > H6, suggesting that surfactant type affects the final mechanical properties of the bigel. A similar trend was observed in the storage modulus values presented in
Figure 4 and
Table 2. The studied bigels can be referred to as emulsion-filled gels, consisting of structured oil droplets emulsified in a gelatin gel matrix. The mechanical properties of such systems depend on the physiochemical properties of the continuous gel matrix, i.e., gelatin hydrogel, the emulsified structured oil droplets hardness, volume fraction, droplet size and distribution, i.e., oleogel, and the interactions between the two phases [
57,
58]. According to the bigel preparation procedure, the volume of the oil fraction is constant for all of the samples but according to the microscopy analysis, droplet size, distribution, and content varied among the different samples. Droplet size analysis suggests a negative relationship between droplet size and bigel hardness according to which hardness increases with the decrease in droplet size. Kim et al. [
59] studied agar-based emulsion gels with different oil droplet size and found that smaller droplets resulted in harder gels. McClements et al. [
60] showed the same trend in their examination of corn oil droplets dispersed in whey protein hydrogel.
Different theories have been developed to describe the effect of particle/droplet type and interfacial relations with continuous gel phase on the final gel properties. Theories introduced by Van der Poel and Kerner suggested that the hardness of the filler, i.e., droplet or particle, affects the hardness of the final gel [
61]. Oliver et el. [
58] examined different oil phase sources, with different saturated fat contents, in the hydrogel phase, in order to study the effect of oil droplet hardness on the textural characteristics of the emulsion. They found that harder droplets resulted in higher emulsion fracture stress, which implies a harder emulsion gel. To examine this theory, the hardness of an oil phase containing 25 wt % GMS and 2 wt % SEs with different HLB values was measured (
Table 4). According to the results, oleogels prepared with SP30 produced harder gels compared with oleogels prepared with SP01. Assuming that oleogels behave as active fillers due to the use of surfactants and, due to the fact that at the studied concentration (1 wt %), gelatin hydrogels produce significantly softer gels compared with the oleogels [
19], filler particles deform less than the continuous hydrogel matrix. Thus, the oleogel droplets are expected to have a stronger impact on bigel properties [
61]. Our results show the opposite trend, with higher hardness values for bigel systems prepared with the softer oleogel formulation (
Table 4). This behavior suggests a stronger effect of droplet size on the bigel’s mechanical properties compared with filler hardness. Another explanation of these results refers to the differences in emulsifier composition at the oil–water interface, resulting in different contents of structuring agent in the oil phase. As discussed above in
Section 3.4, we suspect that the water–oil interface in the H6 sample contained higher amounts of SEs compared with the SEs content in the interfaces of the H1 and H2 samples due to the high content of mono-esterified SEs. Therefore, it is assumed that the oil phase in samples H1 and H2 will contain more SEs compared with sample H6. As described previously, SEs can act as oil-structuring agents and lead to oil solidification and higher hardness [
34]. Thus, higher SE content in the oil phase of samples H1 and H2 will lead to higher hardness values for the oil phase, resulting in higher bigels hardness.
Cohesiveness values, which describe the internal bonds within the sample or substance that resist mastication before breaks [
62], show no significant difference between the formulations. Previous studies done on emulsions prepared using various surfactants with various HLB values concluded that only surfactant concentration, and not the surfactant’s HLB value, has any significant effect on the cohesiveness of the emulsion [
63].
The spreadability test results are in line with the TPA with respect to firmness and spreadability (
Table 4). Spreadability analysis is performed using a 90-degree cone that penetrates into an equivalent cup filled with the sample. The test provides an indication of the sample’s spreadability in compression mode. Firmness, defined as the maximum force during the penetration, exhibits a trend similar to that determined by the TPA for hardness. The spreadability parameter, defined as the area under the curve of the penetration process, is indicative of how easy it will be to spread the sample: the lower the calculated value, the easier it will be to spread [
64]. The results show a positive relationship between sample hardness and spreadability, whereby the hardest formulation, H1, demonstrated the highest spreadability value and the softest formulation, H6, exhibited the lowest spreadability value. The harder bigel exhibited higher loads throughout the entire test, resulting in higher work during cone penetration.
3.7. Flow Behavior
Emulsion viscosity is an important characteristic that can influence the production process and properties of the final product (such as the ability to hold air and stabilize foams, and the tendency to separate) [
10].
Figure 6 presents the viscosity vs. shear rate curves obtained for the emulsions. The mass fraction of the oil phase is 0.5 (
Table 1), and so the volume fraction is higher than 0.5 (due to the higher density of the water phase compared with the oil phase). Such a high-volume fraction results in a flocculated system, in which droplets are clustered and interact with neighboring droplets, as seen in
Figure 5. Berli, Quemada, and Parker [
65] described such a system as a glassy system, in which droplets are trapped in transient cages formed by their nearest neighbors. Such a system can exhibit both solid-like behavior at low shear rates and liquid-like behavior at high shear rates.
The decrease in viscosity with the increase in shear rate, as observed in
Figure 6, defines a pseudoplastic behavior. The most popular model to fit a wide range of shear rates of pseudoplastic systems is the Carreau model [
66]
where
is the measured viscosity,
is the viscosity at low shear rates,
is the viscosity at high shear rates,
is the shear rate,
is the relaxation time, and n is the power index. This model describes three main flow regimes according to which a pseudoplastic system will flow. In the initial regime, at very low shear rates, viscosity is constant and equal to
. The lower regime shear rate used in the current study, 0.01 s
−1, was not low enough to detect this regime. In the second regime, at intermediate shear rates, 0.01 to 1 s
−1, the critical yield stress is exceeded and particles can move past one another, leading to a decrease in the observed viscosity. This region can be seen in
Figure 6B and was fitted to a power-law, which can be derived from the Carreau model [
67]
where k is referred to as consistency index and is the viscosity at zero shear rates [
2].
Table 5 presents the fitted parameters. The slope, defined as (n−1), provides n values of 0.20 ± 0.03, 0.19 ± 0.05, and 0.22 ± 0.03 for H1, H2, and H6, respectively. These values support our assumption claiming pseudoplastic behavior, which is characterized by n < 1. Moran-Valero et al. [
11] examined the effect of different ratios of lecithin and GMS on the viscosity of O/W emulsions and showed that when lecithin dominates the flow, emulsions exhibit highly pseudoplastic behavior with n = 0.29 [
11,
67]. On the other hand, when GMS is present in the oil–water interface, Newtonian behavior (n = 1) is observed [
68]. They also showed that when the concentration of lecithin is lower than that of GMS, such as in the current studied systems, the rheological behavior is mostly affected by GMS, making the emulsion less pseudoplastic, thus increasing the value of n. The calculated n values in the current research (
Table 5) suggest emulsion gel systems in which lecithin dominates the flow; thus, most of the GMS remains in the oil droplets and not in the oil–water interface, as previously suggested by the microscopy analysis (
Section 3.4). It is important to note that even though the n value of H6 was found to be statistically different, it is very close to the n values for H1 and H2, and so it can be assumed that the degree of SE esterification did not have a large effect on the pseudoplastic behavior.
Interestingly, a change in the plot slope was seen around shear rate 2.51 s
−1 (marked by a dashed horizontal line), and this change was more significant for the H6 samples than for the H1 and H2 samples. Such a change in the viscosity slope can imply changes in the flow behavior of the oleogel droplets. More specifically, the H6 curve exhibited a significant decrease in viscosity, which led to an increase in the slope. This sharp decline can be the result of droplet deformation caused by shear forces. In the intermediate shear rates, the velocity gradient is high enough to separate and disrupt the flocs but not high enough as to cause deformation of the droplets. At higher shear rates, the velocity gradient caused by the high shear rate is high enough to deform the droplets; thus, the droplets no longer remain spherical and viscosity decreases more sharply. Otsubo and Prud’homme [
69] studied the effects of droplet deformation on viscosity. They claimed that there is a critical droplet deformation index above which the droplets will be broken by the shear forces, and as a result, the coalescence and breakup of droplets will be induced. This breakup and coalescence of droplets results in an increase in droplet size, in turn resulting in a change in the slope of the viscosity curve and in a sharper decrease in viscosity.
According to the third regime, at high shear rates, viscosity reaches a second plateau that is characterized by a constant viscosity and is defined as
. At these high shear rates, the droplets/particle flocs separate, and the droplets deform and move past one another. This regime was only observed for the H6 sample at shear rates higher than 10 s
−1. The fact that the third region and the significant slope change were observed for the H6 sample suggests that the droplets in H6 are more sensitive to shear forces, as already shown in the frequency sweep measurements (
Figure 4 and
Table 2), where H6 bigel exhibited a more frequency-dependent behavior. This phenomenon also supports our assumption that when HLB6 SEs are used, the oil–water interface will contain more SEs compared with the bulk oil, as opposed to bigels with HLB1 and HLB2 SEs, in which SEs are located mainly in the bulk oil, thus strengthening the oleogel network within the oil phase to a lesser degree, resulting in higher sensitivity of the oil droplets to shear forces.