Lime Juice Enhances Calcium Bioaccessibility from Yogurt Snacks Formulated with Whey Minerals and Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid Chromatographic Analysis of Citric Acid in Lime Juice
2.3. Yogurt Base Preparation
2.4. Freeze Drying and Snack Composition
2.5. In Vitro Static Digestion
2.6. Calcium Analysis
2.6.1. Free Calcium
2.6.2. Total Calcium
2.6.3. Soluble Calcium
2.7. Calcium Binding Measurements
2.8. Dissolution of WMC by Lime Juice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Snacks
3.2. Calcium Analysis
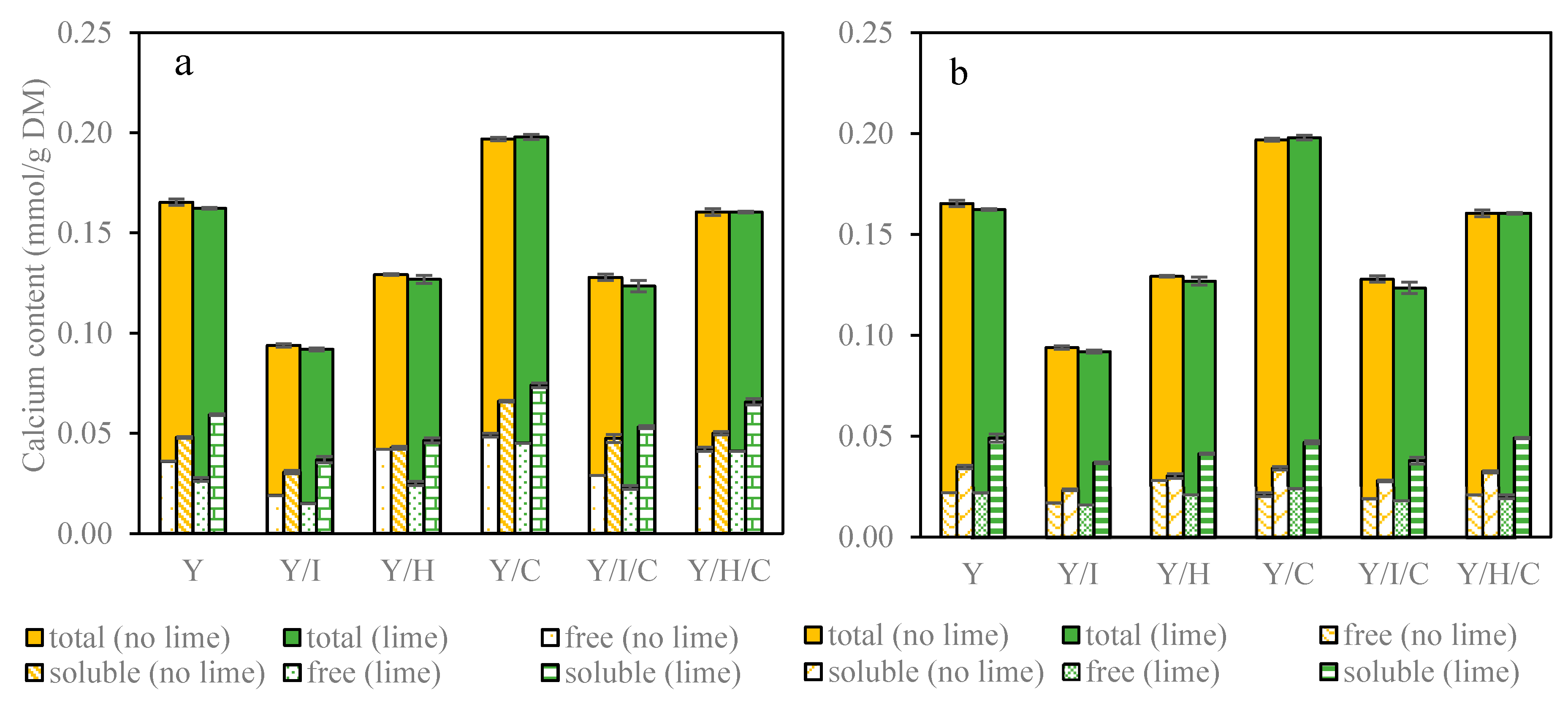
3.2.1. Total Calcium Concentration of Yogurt Snacks
3.2.2. Free Calcium Concentration during In Vitro Digestion
3.2.3. Soluble Calcium Concentration and Calcium Bioaccessibility
3.3. Calcium Binding Measurements at the Beginning of Intestinal Digestion
3.4. Dissolution of WMC by Lime Juice in Presence of WPI or WPH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Graaf, C. Effects of snacks on energy intake: An evolutionary perspective. Appetite 2006, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Maetens, E.; Hettiarachchy, N.; Dewettinck, K.; Horax, R.; Moens, K.; Moseley, D.O. Physicochemical and nutritional properties of a healthy snack chip developed from germinated soybeans. LWT Food Sci. Technol. 2017, 84, 505–510. [Google Scholar] [CrossRef]
- Njike, V.Y.; Smith, T.M.; Shuval, O.; Shuval, K.; Edshteyn, I.; Kalantari, V.; Yaroch, A.L. Snack food, satiety, and weight. Adv. Nutr. 2016, 7, 866–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unal, G.; El, S.N.; Kilic, S. In vitro determination of calcium bioavailability of milk, dairy products and infant formulas. Int. J. Food Sci. Nutr. 2005, 56, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.P.; Evans, E.M. Dietary protein and bone health: Harmonizing conflicting theories. Nutr. Rev. 2011, 69, 215–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pr. 2007, 22, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M.Q.; Rubió, J.B.; Curiel, M.D.; Pérez, A.D. Calcium citrate and vitamin D in the treatment of osteoporosis. Clin. Drug Investig. 2011, 31, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.C.; Harvey, J.A.; Hsu, M.C. Enhanced calcium bioavailability from a solubilized form of calcium citrate. J. Clin. Endocrinol. Metab. 1987, 65, 801–805. [Google Scholar] [CrossRef]
- De Zawadzki, A.; Skibsted, L.H. Increasing calcium solubility from whey mineral residues by combining gluconate and δ-gluconolactone. Int. Dairy J. 2019, 99, 104538. [Google Scholar] [CrossRef]
- De Zawadzki, A.; Skibsted, L.H. Calcium availability from whey mineral residues increased by hydrogen citrate. Food Res. Int. 2020, 137, 109372. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Barbé, F.; Ménard, O.; Le Gouar, Y.; Buffière, C.; Famelart, M.-H.; Laroche, B.; Le Feunteun, S.; Dupont, D.; Rémond, D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem. 2013, 136, 1203–1212. [Google Scholar] [CrossRef]
- Wang, J.; Aalaei, K.; Skibsted, L.H.; Ahrné, L. Bioaccessibility of calcium in freeze-dried yogurt based snacks. LWT Food Sci. Technol. 2020, 129, 109527. [Google Scholar] [CrossRef]
- Nooshkam, M.; Madadlou, A. Microwave-assisted isomerisation of lactose to lactulose and Maillard conjugation of lactulose and lactose with whey proteins and peptides. Food Chem. 2016, 200, 1–9. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Whey protein peptides as components of nanoemulsions: A review of emulsifying and biological functionalities. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- Wang, S.; Lina, Z.; Shaoyun, W.; Pingfan, R. Fabrication and characterization of the nano-composite of whey protein hydrolysate chelated with calcium. Food Funct. 2015, 6, 816–823. [Google Scholar]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Morr, C.; German, B.; Kinsella, J.; Regenstein, J.; Van Buren, J.; Kilara, A.; Lewis, B.; Mangino, M. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Vavrusova, M.; Skibsted, L.H. Aqueous solubility of calcium citrate and interconversion between the tetrahydrate and the hexahydrate as a balance between endothermic dissolution and exothermic complex formation. Int. Dairy J. 2016, 57, 20–28. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N.; Tianco, E.M.; Loy, T.A. Ionic strengths of the solutions of flooded soils and other natural aqueous solutions from specific conductance. Soil Sci. 1966, 102, 408–413. [Google Scholar] [CrossRef]
- Lorieau, L.; Le Roux, L.; Gaucheron, F.; Ligneul, A.; Hazart, E.; Dupont, D.; Floury, J. Bioaccessibility of four calcium sources in different whey-based dairy matrices assessed by in vitro digestion. Food Chem. 2018, 245, 454–462. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium binding to amino acids and small glycine peptides in aqueous solution: Toward peptide design for better calcium bioavailability. J. Agric. Food Chem. 2016, 64. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Water Activity in Foods: Fundamentals and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Skibsted, L.H. Mineral nutrient interaction: Improving bioavailability of calcium and iron. Food Sci. Biotechnol. 2016, 25, 1233–1241. [Google Scholar] [CrossRef]
- Goss, S.L.; Lemons, K.A.; Kerstetter, J.E.; Bogner, R.H. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol. 2007, 59. [Google Scholar] [CrossRef] [PubMed]
- Vavrusova, M.; Skibsted, L.H. Calcium nutrition. Bioavailability and fortification. LWT Food Sci. Technol. 2014, 59, 1198–1204. [Google Scholar] [CrossRef]
- Garcia, A.C.; Vavrusova, M.; Skibsted, L.H. Supersaturation of calcium citrate as a mechanism behind enhanced availability of calcium phosphates by presence of citrate. Food Res. Int. 2018, 107, 195–205. [Google Scholar] [CrossRef]
- Walser, M. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J. Clin. Investig. 1961, 40, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R. Calcium: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Smialowska, A.; Merino, L.M.-M.; Ingham, B.; Carr, A. Effect of calcium on the aggregation behaviour of caseinates. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 113–123. [Google Scholar] [CrossRef]





| Sample | Yogurt | Sucrose | Pectin Solution (2.0%) | WPI Solution (8.0%) | WPH Solution (8.0%) | WMC Solution (1.4%) | H2O | Lime Juice | pH |
|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | / | |
| Y | 70 | 10 | 10 | / | / | / | 10 | / | 4.29 ± 0.01 * |
| Y/L | 70 | 10 | 10 | / | / | / | 7 | 3 | 4.01 ± 0.00 * |
| Y/I | 35 | 10 | 10 | 35 | / | / | 10 | / | 4.83 ± 0.03 * |
| Y/I/L | 35 | 10 | 10 | 35 | / | / | 7 | 3 | 4.29 ± 0.01 * |
| Y/H | 35 | 10 | 10 | / | 35 | / | 10 | / | 4.93 ± 0.02 * |
| Y/H/L | 35 | 10 | 10 | / | 35 | / | 7 | 3 | 4.44 ± 0.02 * |
| Y/C | 70 | 10 | 10 | / | / | 7 | 3 | / | 4.41 ± 0.01 * |
| Y/C/L | 70 | 10 | 10 | / | / | 7 | / | 3 | 4.13 ± 0.00 * |
| Y/I/C | 35 | 10 | 10 | 35 | / | 7 | 3 | / | 4.98 ± 0.02 * |
| Y/I/C/L | 35 | 10 | 10 | 35 | / | 7 | / | 3 | 4.43 ± 0.01 * |
| Y/H/C | 35 | 10 | 10 | / | 35 | 7 | 3 | / | 5.09 ± 0.03 * |
| Y/H/C/L | 35 | 10 | 10 | / | 35 | 7 | / | 3 | 4.57 ± 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Aalaei, K.; Skibsted, L.H.; Ahrné, L.M. Lime Juice Enhances Calcium Bioaccessibility from Yogurt Snacks Formulated with Whey Minerals and Proteins. Foods 2020, 9, 1873. https://doi.org/10.3390/foods9121873
Wang J, Aalaei K, Skibsted LH, Ahrné LM. Lime Juice Enhances Calcium Bioaccessibility from Yogurt Snacks Formulated with Whey Minerals and Proteins. Foods. 2020; 9(12):1873. https://doi.org/10.3390/foods9121873
Chicago/Turabian StyleWang, Jing, Kataneh Aalaei, Leif H. Skibsted, and Lilia M. Ahrné. 2020. "Lime Juice Enhances Calcium Bioaccessibility from Yogurt Snacks Formulated with Whey Minerals and Proteins" Foods 9, no. 12: 1873. https://doi.org/10.3390/foods9121873
APA StyleWang, J., Aalaei, K., Skibsted, L. H., & Ahrné, L. M. (2020). Lime Juice Enhances Calcium Bioaccessibility from Yogurt Snacks Formulated with Whey Minerals and Proteins. Foods, 9(12), 1873. https://doi.org/10.3390/foods9121873






