Optical Absorption and Scattering Properties at 900–1650 nm and Their Relationships with Soluble Solid Content and Soluble Sugars in Apple Flesh during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Storage
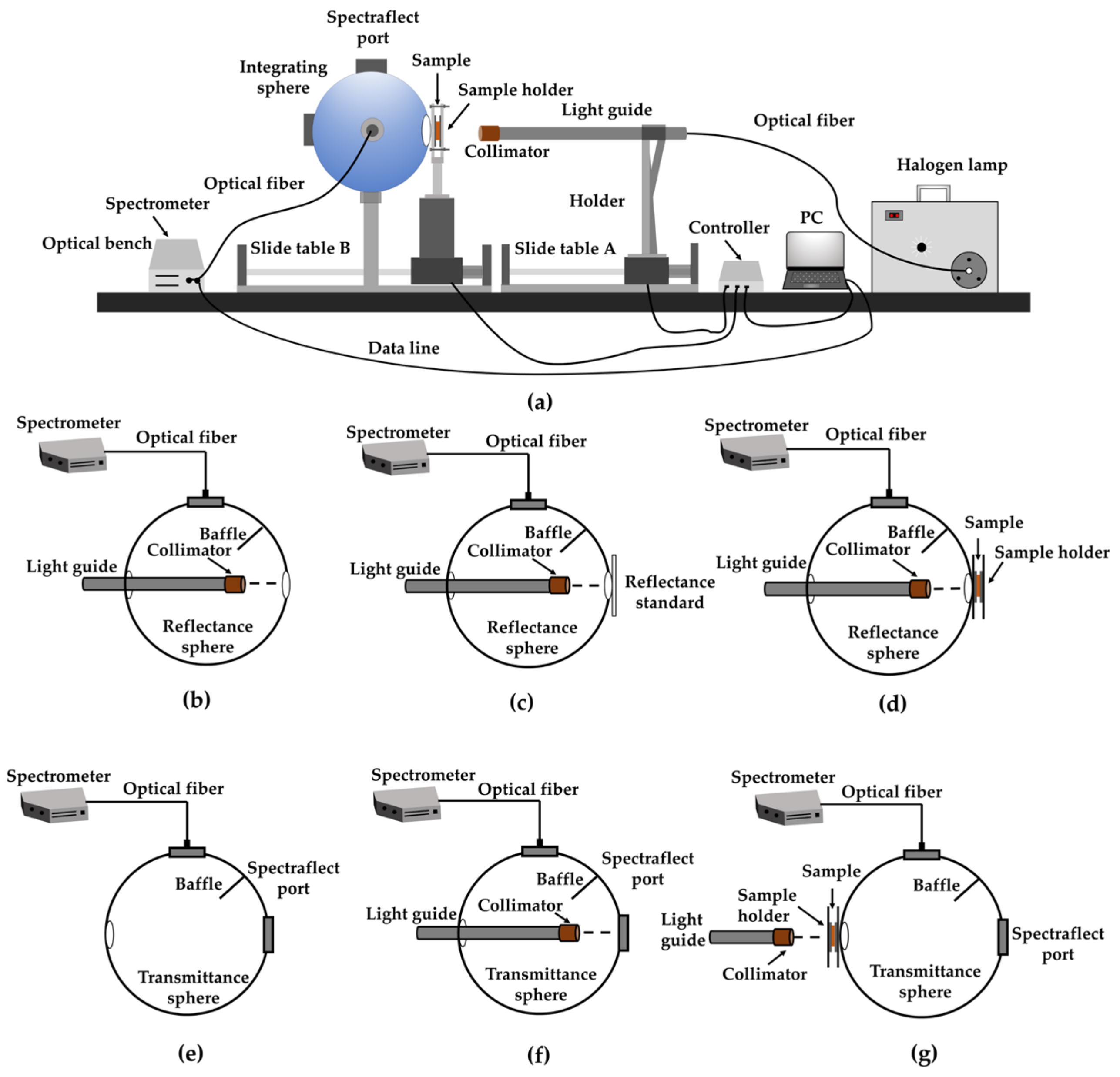
2.2. Optical Properties
2.3. SSC and Soluble Sugars Contents Measurements
2.4. Statistical Analysis
3. Results and Discussion
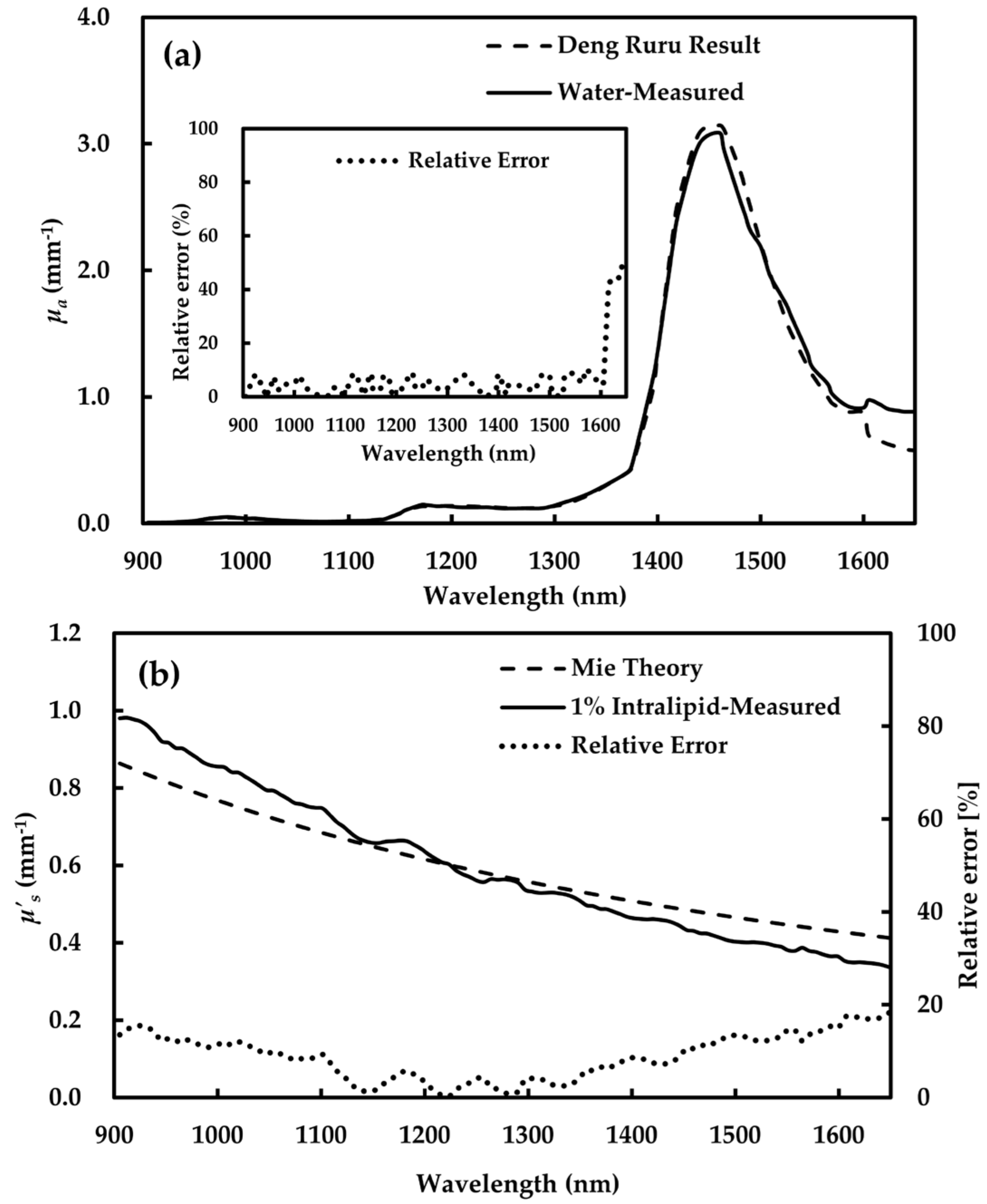
3.1. Validation Results of Optical Properties
3.2. Spectral Analysis
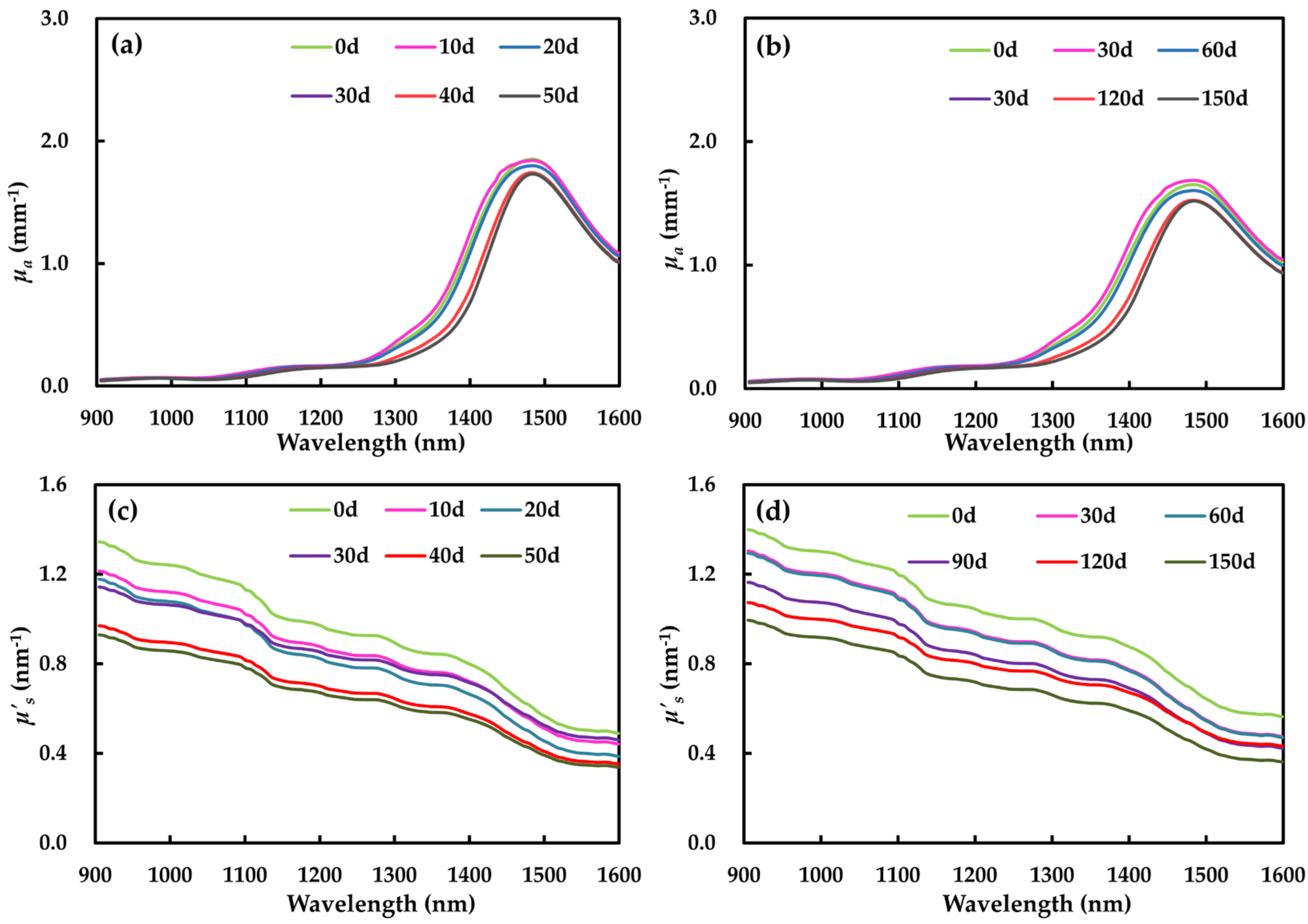
3.2.1. Absorption Coefficient
3.2.2. Reduced Scattering Coefficient
3.3. SSC and Soluble Sugars Contents
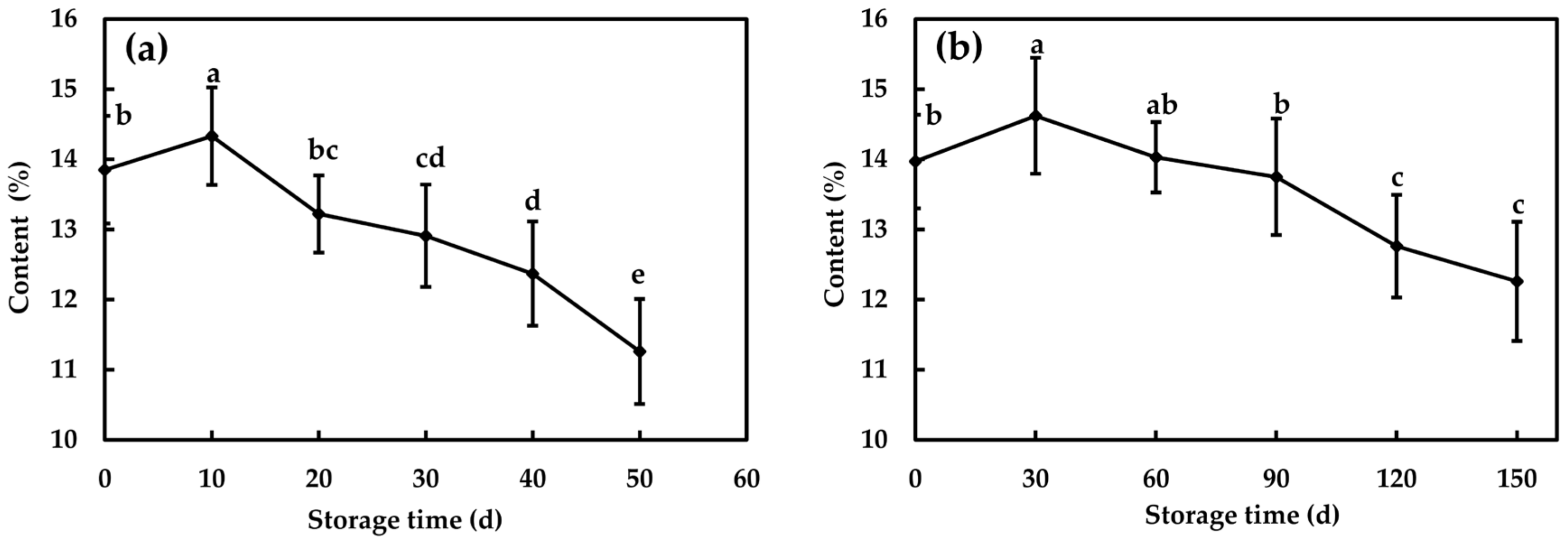
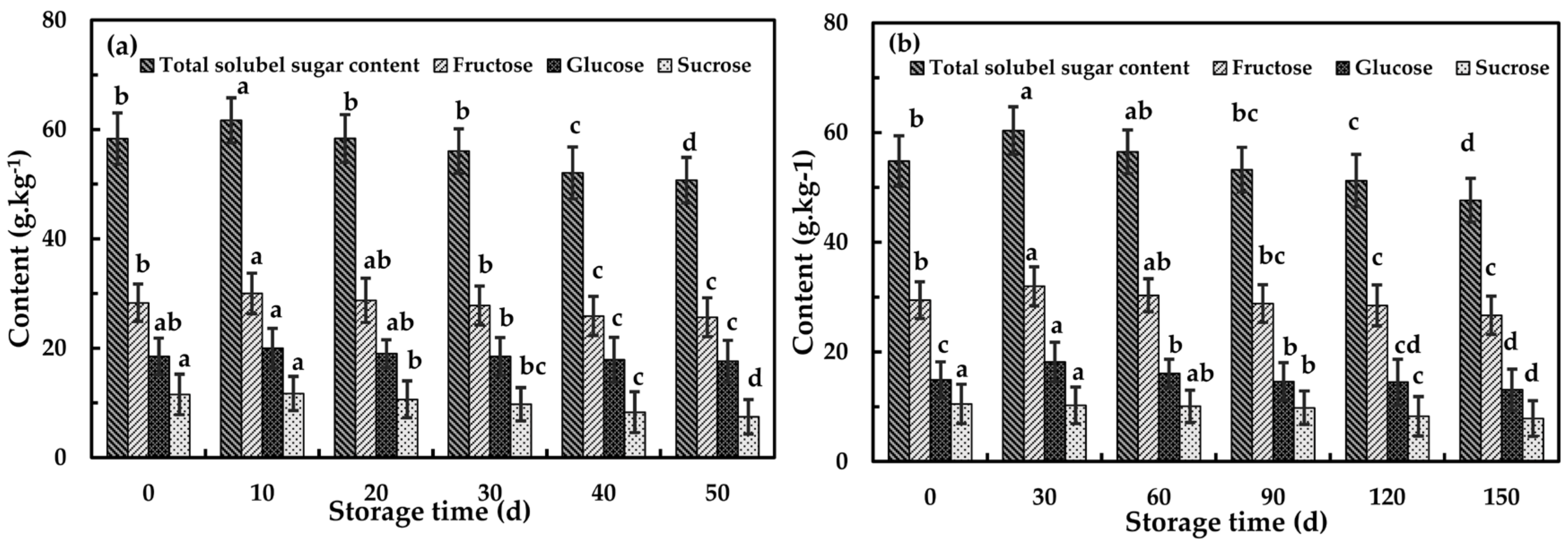
3.3.1. Changes of Contents of Soluble Solid and Soluble Sugars
3.3.2. Relationships among SSC and Soluble Sugar Contents
3.4. Optical Properties–SSC–Soluble Sugars Contents Relations
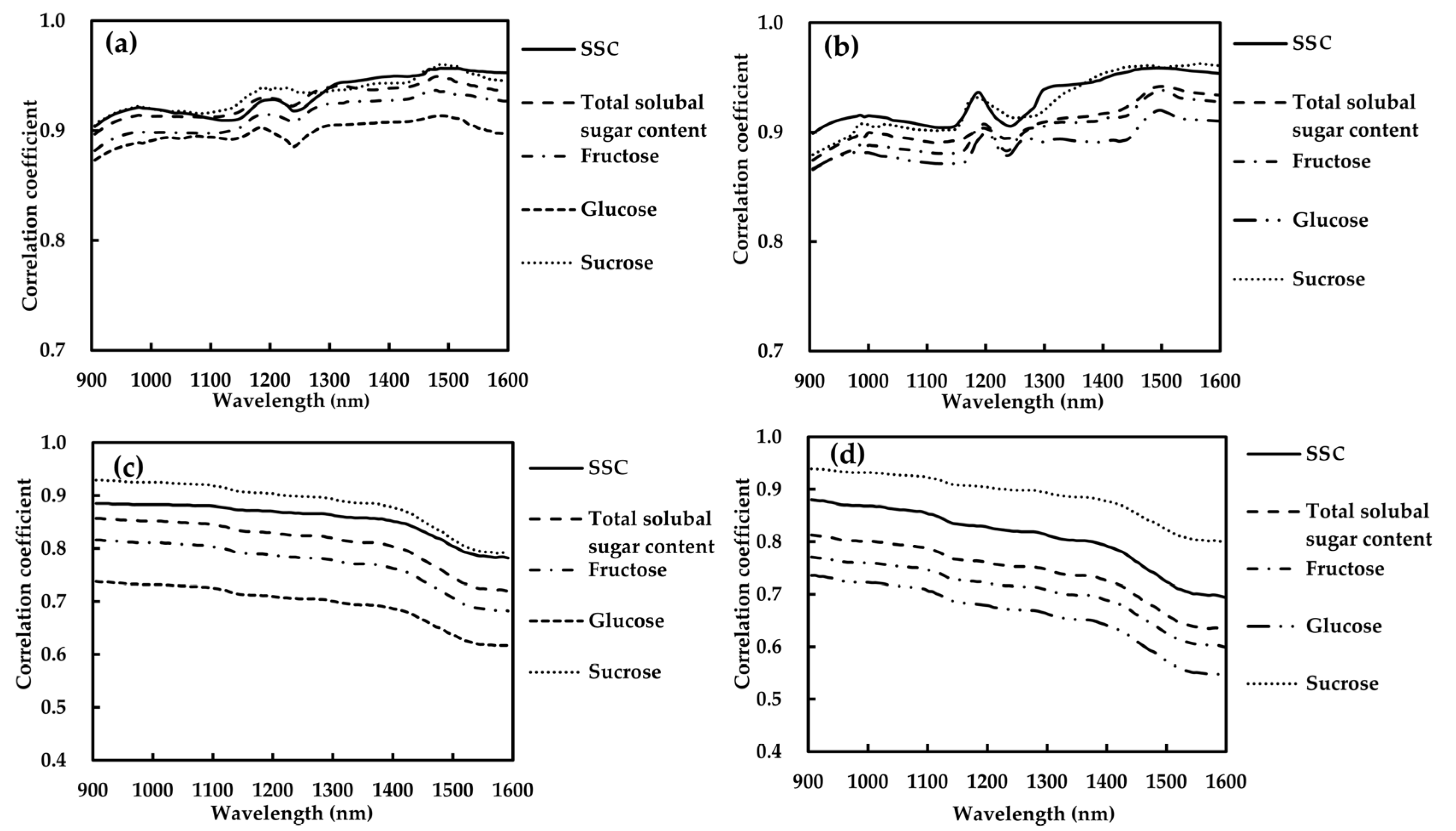
3.4.1. Absorption Coefficient–SSC–Soluble Sugars Contents Relations
3.4.2. Scattering Coefficient–SSC–Soluble Sugars Contents Relations
3.5. SSC and Soluble Sugars Contents Predictions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, M.J.; Feng, F.J.; Cheng, L.L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, 33055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; He, J.; Zheng, S.; Ye, D. Modeling for SSC and firmness detection of persimmon based on NIR hyperspectral imaging by sample partitioning and variables selection. Infrared Phys. Technol. 2019, 103099. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhan, B.; Wang, Z.; Jiang, Y. Determination of SSC in pears by establishing the multi-cultivar models based on visible-NIR spectroscopy. Infrared Phys. Technol. 2019, 102, 103006. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Q.; Tian, X.; Yang, G.; Xia, Y.; Li, J.; Huang, W. Non-destructive evaluation of soluble solids content of apples using a developed portable Vis/NIR device. Biosyst. Eng. 2020, 198, 138–148. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.M.; Agyekum, A.A.; Wu, J.J.; Chen, Q.S.; Zuo, M. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, K.L.; Liu, Q.; Pan, L.; Tu, K. Classification and discrimination of different fungal diseases of three infection levels on peaches using hyperspectral reflectance imaging analysis. Sensors 2018, 14, 1295. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wei, K.L.; Xiao, H.; Tu, S.C.; Sun, K.; Sun, Y.; Pan, L.Q.; Tu, K. Near-infrared hyperspectral imaging rapidly detects the decay of postharvest strawberry based on water-soluble sugar analysis. Food Anal. Methods 2019, 12, 936–946. [Google Scholar] [CrossRef]
- Valero, C.; Ruizaltisent, M.; Cubeddu, R.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Johnson, D.S.; Dover, C.J. Detection of internal quality in kiwi with time-domain diffuse reflectance spectroscopy. Appl. Eng. Agric. 2004, 20, 223–232. [Google Scholar] [CrossRef]
- Oliveira-Folador, G.; Bicudo, M.D.O.; de Andrade, E.F.; Renard, C.M.-G.C.; Bureau, S.; de Castilhos, F. Quality traits prediction of the passion fruit pulp using NIR and MIR spectroscopy. LWT Food Sci. Technol. 2018, 95, 172–178. [Google Scholar] [CrossRef]
- Adebayo, S.E.; Hashim, N.; Abdan, K.; Hanafi, M.; Mollazade, K. Prediction of quality attributes and ripeness classification of bananas using optical properties. Hortic. Sci. 2016, 212, 171–182. [Google Scholar] [CrossRef]
- Mo, C.Y.; Kim, M.S.; Kim, G.; Lim, J.; Delwiche, S.R.; Chao, K.L.; Li, H.; Cho, B.-K. Spatial assessment of soluble solid contents on apple slices using hyperspectral imaging. Biosyst. Eng. 2017, 159, 10–21. [Google Scholar] [CrossRef]
- Pan, L.Q.; Lu, R.F.; Zhu, Q.B.; McGrath, J.M.; Tu, K. Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2015, 102, 42–50. [Google Scholar] [CrossRef]
- Xie, D.D.; Guo, W.C. Measurement and Calculation Methods on Absorption and Scattering Properties of Turbid Food in Vis/NIR Range. Food Bioprocess Technol. 2020, 13, 229–244. [Google Scholar] [CrossRef]
- Lu, R.F.; Van, B.R.; Saeys, W.; Li, C.; Cen, H. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biol. Technol. 2020, 159, 111003. [Google Scholar] [CrossRef]
- Hu, D.; Fu, X.P.; Wang, A.C.; Ying, Y.B. Measurement methods for optical absorption and scattering properties of fruits and vegetables. Trans. ASABE 2015, 58, 1387–1401. [Google Scholar] [CrossRef]
- Tuchin, V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis; SPIE Press: Bellingham, WA, USA, 2007. [Google Scholar]
- Pickering, J.W. Double-integrating-sphere system for measuring the optical properties of tissue. Appl. Opt. 1993, 32, 399–410. [Google Scholar] [CrossRef]
- López Maestresalas, A.; Aernouts, B.; Van Beers, R.; Arazuri, S.; Jarén, C.; De Baerdemaeker, J.; Saeys, W. Bulk optical properties of potato flesh in the 500–1900 nm range. Food Bioprocess Technol. 2015, 9, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.D.T.; Erkinbaev, C.; Tsuta, M.; De Baerdemaeker, J.; Nicolaï, B.; Saeys, W. Spatially resolved diffuse reflectance in the visible and near-infrared wavelength range for non-destructive quality assessment of ‘Braeburn’ apples. Postharvest Biol. Technol. 2014, 91, 39–48. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Farina, A.; Pifferi, A.; Spinelli, L.; Torricelli, A. Time-resolved reflectance spectroscopy nondestructively reveals structural changes in ‘Pink Lady®’ apples during storage. Procedia Food Sci. 2011, 1, 81–89. [Google Scholar] [CrossRef] [Green Version]
- He, X.M.; Fu, X.P.; Li, T.W.; Rao, X.Q. Spatial frequency domain imaging for detecting bruises of pears. J. Food Meas. Charact. 2018, 12, 1266–1273. [Google Scholar] [CrossRef]
- Prahl, S.A.; Martin, J.C.; Germet, V.; Welch, A.J. Determining the optical properties of turbid media by using the adding-doubling method. Appl. Opt. 1993, 32, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Jacques, S.L.; Zheng, L.Q. Conv-convolution for responses to a finite diameter photon beam incident on multi-layered tissues. Comput. Meth. Prog. Biol. 1997, 54, 141–150. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, C.Y.; Yang, F.Z. Optical properties of blueberry flesh and skin and Monte Carlo multi-layered simulation of light interaction with fruit tissues. Postharvest Biol. Technol. 2019, 150, 28–41. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lu, R.F.; Chen, K. Assessment of Tomato Soluble Solids Content and pH by Spatially-Resolved and Conventional Vis/NIR Spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Rowe, P.I.; Künnemeyer, R.; McGlone, A.; Talele, S.; Martinsen, P.; Seelye, R. Relationship between tissue firmness and optical properties of ‘Royal Gala’ apples from 400 to 1050 nm. Postharvest Biol. Technol. 2014, 94, 89–96. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, X.H.; Zhang, S.H.; Cheng, Q.L.; Tan, Z.J. An effective method to inspect and classify the bruising degree of apples based on the optical properties. Postharvest Biol. Technol. 2017, 127, 44–52. [Google Scholar] [CrossRef]
- He, X.M.; Fu, X.P.; Rao, X.Q.; Fang, Z.H. Assessing firmness and SSC of pears based on absorption and scattering properties using an automatic integrating sphere system from 400 to 1150 nm. Postharvest Biol. Technol. 2016, 121, 62–70. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Gitaitis, R. Optical properties of healthy and diseased onion tissues in the visible and near-infrared spectral region. Trans. ASABE 2014, 57, 1771–1782. [Google Scholar] [CrossRef]
- Vanoli, M.; Zerbini, P.; Spinelli, L.; Torricelli, A.; Rizzolo, A. Polyuronide content and correlation to optical properties measured by time-resolved reflectance spectroscopy in ‘Jonagored’ apples stored in normal and controlled atmosphere. Food Chem. 2009, 115, 1450–1457. [Google Scholar] [CrossRef]
- Cen, H.Y.; Lu, R.F.; Mendoza, F.A.; Beaudry, R.M. Relationship of the optical absorption and scattering properties with mechanical and structural properties of apple tissue. Postharvest Biol. Technol. 2013, 85, 30–38. [Google Scholar] [CrossRef]
- Hjalmarsson, P.; Thennadil, S.N. Spatially resolved in vivo measurement system for estimating the optical properties of tissue in the wavelength range 1000–1700 nm. In Proceedings of SPIE the International Society for Optical Engineering; Schweitzer, D., Fitzmaurice, M., Eds.; SPIE: Bellingham, DC, USA, 2007; Volume 6628, p. 662805. [Google Scholar]
- Saeys, W.; Velazco-Roa, M.A.; Thennadil, S.N.; Ramon, H.; Nicolaï, B.M. Optical properties of apple skin and flesh in the wavelength range from 350 to 2200 nm. Appl. Opt. 2008, 47, 908–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.H.; Nadeau, K.P.; Jaworski, F.B.; Tromberg, B.J.; Durkin, A.J. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Opt. 2015, 20, 30901. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, X.Z.; Inagaki, T.; Yang, H.; Tsuchikawa, S. Noncontact evaluation of soluble solids content in apples by near-infrared hyperspectral imaging. J. Food Eng. 2018, 224, 53–61. [Google Scholar] [CrossRef]
- Wei, K.L.; Ma, C.; Sun, K.; Liu, Q.; Zhao, N.; Sun, Y.; Tu, K.; Pan, L. Relationship between optical properties and soluble sugar contents of apple flesh during storage. Postharvest Biol. Technol. 2020, 159, 111021. [Google Scholar] [CrossRef]
- Prahl, S.A. Everything I Think You Should Know about Inverse Adding-Doubling. Available online: https://omlc.ogi.edu/software/iad (accessed on 12 March 2019).
- Van Staveren, H.J.; Moes, C.J.; Van, M.J. Light scattering in Intralipid-10% in wavelength range of 400–1100 nm. Appl. Opt. 1991, 30, 4507–4514. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.R.; He, Y.Q.; Qin, Y.; Chen, Q.D. Measuring pure water absorption coefficient in the near-infrared spectrum (900–2500 nm). J. Remote Sens. 2012, 16, 192–206. [Google Scholar] [CrossRef]
- Ma, C.; Feng, L.; Pan, L.; Wei, K.; Liu, Q.; Tu, K.; Zhao, L.; Peng, J. Relationships between optical properties of peach flesh with firmness and tissue structure during storage. Postharvest Biol. Technol. 2020, 163, 111134. [Google Scholar] [CrossRef]
- Van, B.R.; Aernouts, B.; Watte, R.; Schenk, A.; Nicolaï, B.; Saeys, W. Effect of maturation on the bulk optical properties of apple skin and cortex in the 500–1850 nm wavelength range. J. Food Eng. 2017, 214, 79–89. [Google Scholar] [CrossRef]
- Fang, Z.H.; Fu, X.P.; He, X.M. Investigation of absorption and scattering characteristics of kiwifruit tissue using a single integrating sphere system. J. Zhejiang Univ. Sci. B 2016, 17, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Torricelli, A.; Valentini, G.; Johnson, D. Time-resolved reflectance spectroscopy applied to the nondestructive monitoring of the internal optical properties in apples. Appl. Spectrosc. 2001, 55, 1368–1374. [Google Scholar] [CrossRef] [Green Version]
- Blažek, J.; Hlušičková, I.; Varga, A. Changes in quality characteristics of Golden Delicious apples under different storage conditions and correlations between them. Hortic. Sci. 2018, 30, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Davirai, M.; Helene, H.; Philip, R.; Eyéghè-Bickong, H.A.; Vivier, M.A. A rapid qualitative and quantitative evaluation of grape berries at various stages of development using Fourier-transform infrared spectroscopy and multivariate data analysis. Food Chem. 2016, 190, 253–262. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Zerbini, P.E.; Schouten, R.E.; Vanoli, M.; Jacob, S.; Grassi, M. Assessing harvest maturity in nectarines. Postharvest Biol. Technol. 2007, 45, 204–213. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Xiong, Y.; Tan, X.; Zhang, B. Determination of soluble solid content in multi-origin ‘Fuji’ apples by using FT-NIR spectroscopy and an origin discriminant strategy. Comput. Electron. Agric. 2018, 155, 23–31. [Google Scholar] [CrossRef]
| SSC | TSS | Fructose | Glucose | Sucrose | |
|---|---|---|---|---|---|
| SSC | 1 | 0.956 ** | 0.921 ** | 0.868 * | 0.973 ** |
| TSS | \ | 1 | 0.974 ** | 0.942 ** | 0.921 ** |
| Fructose | \ | \ | 1 | 0.957 ** | 0.943 ** |
| Glucose | \ | \ | \ | 1 | 0.841 * |
| Sucrose | \ | \ | \ | \ | 1 |
| SSC | TSS | Fructose | Glucose | Sucrose | |
|---|---|---|---|---|---|
| SSC | 1 | 0.963 ** | 0.933 ** | 0.870 * | 0.950 ** |
| TSS | \ | 1 | 0.975 ** | 0.967 ** | 0.847 * |
| Fructose | \ | \ | 1 | 0.982 ** | 0.795 |
| Glucose | \ | \ | \ | 1 | 0.685 |
| Sucrose | \ | \ | \ | \ | 1 |
| Parameters (Independent Variable x) | μa (Dependent Variable y) | Linear Fitting Equation | R2 |
|---|---|---|---|
| SSC | 980 nm | y = 0.0021x + 0.0383 | 0.848 |
| 1189 nm | y = 0.0053x +0.1039 | 0.859 | |
| 1498 nm | y = 0.0581x + 1.0107 | 0.915 | |
| Sucrose | 980 nm | y = 0.0022x + 0.0361 | 0.850 |
| 1189 nm | y = 0.005x + 0.1064 | 0.880 | |
| 1498 nm | y = 0.036x + 1.4102 | 0.921 |
| Parameters | Optical Property | Calibration | Prediction | ||
|---|---|---|---|---|---|
| Rc2 | RMSEC | Rp2 | RMSEP | ||
| SSC | μa | 0.851 | 0.314 | 0.833 | 0.329 |
| μ′s | 0.739 | 0.693 | 0.726 | 0.722 | |
| Total soluble sugar | μa | 0.837 | 2.055 | 0.830 | 2.062 |
| μ′s | 0.710 | 3.657 | 0.701 | 3.699 | |
| Fructose | μa | 0.782 | 1.567 | 0.781 | 1.570 |
| μ′s | 0.658 | 1.935 | 0.635 | 2.216 | |
| Glucose | μa | 0.769 | 1.338 | 0.752 | 1.344 |
| μ′s | 0.611 | 1.821 | 0.593 | 1.906 | |
| Sucrose | μa | 0.860 | 1.032 | 0.851 | 1.047 |
| μ′s | 0.744 | 1.437 | 0.736 | 1.458 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Wei, K.; Feng, L.; Tu, K.; Peng, J.; Wang, J.; Pan, L. Optical Absorption and Scattering Properties at 900–1650 nm and Their Relationships with Soluble Solid Content and Soluble Sugars in Apple Flesh during Storage. Foods 2020, 9, 1881. https://doi.org/10.3390/foods9121881
Fang L, Wei K, Feng L, Tu K, Peng J, Wang J, Pan L. Optical Absorption and Scattering Properties at 900–1650 nm and Their Relationships with Soluble Solid Content and Soluble Sugars in Apple Flesh during Storage. Foods. 2020; 9(12):1881. https://doi.org/10.3390/foods9121881
Chicago/Turabian StyleFang, Li, Kangli Wei, Li Feng, Kang Tu, Jing Peng, Jiahong Wang, and Leiqing Pan. 2020. "Optical Absorption and Scattering Properties at 900–1650 nm and Their Relationships with Soluble Solid Content and Soluble Sugars in Apple Flesh during Storage" Foods 9, no. 12: 1881. https://doi.org/10.3390/foods9121881





