Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods
Abstract
:1. Introduction
2. Occurrence and Importance of Mycotoxins in Foods
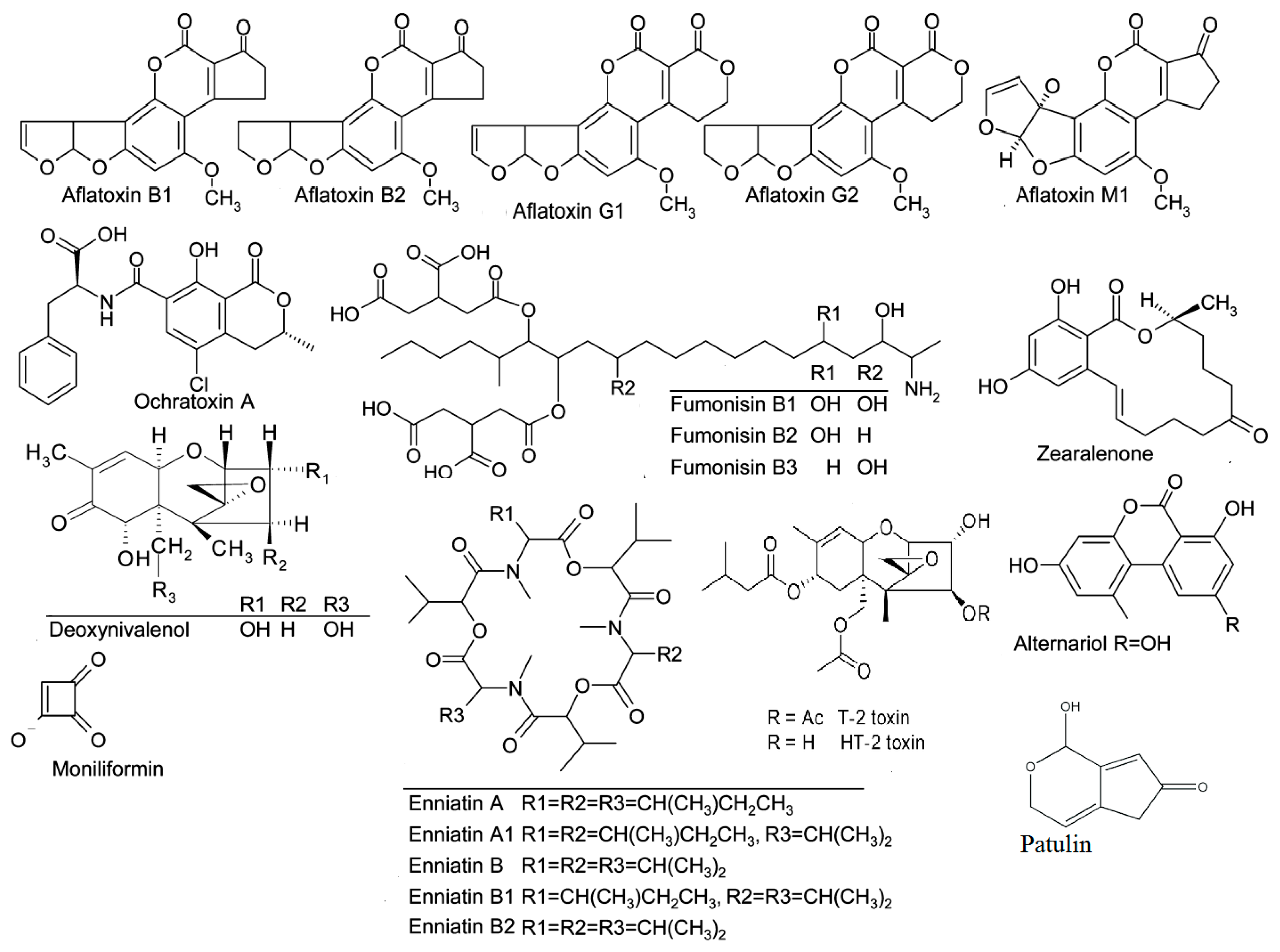
2.1. Aflatoxins
2.2. Ochratoxin A
2.3. Fumonisins
2.4. Trichothecenes
2.4.1. Trichothecenes Type A (HT-2 Toxin and T-2 Toxin)
2.4.2. Trichothecenes Type B (Deoxynivalenol)
2.5. Zearalenone
2.6. Emerging Fusarium Mycotoxins (Fusaproliferin, Moniliformin, Beauvericin, NX-2 Toxin, and Enniatins)
2.7. Ergot Alkaloids
2.8. Alternaria Toxins (Altenuene, Alternariol, Alternariol Methyl Ether, Altertoxin, Tenuazonic Acid, Tentoxin)
2.9. Patulin
3. Mycotoxin Control Strategies: Prevention and Decontamination/Detoxification in Foods
3.1. Pre-Harvest Strategies
3.2. Post-Harvest Strategies
3.2.1. Physical Treatment
Sorting
Processing
Storage
Radiation
Cold Plasma
Mycotoxin Binders
3.2.2. Chemical Control
Bases (Ammonia, Hydrated Oxide)
Chitosan
Ozone Treatment
3.2.3. Biological Control
Bacteria
Yeast
Food Fermentation
Fungi
3.2.4. Enzymatic Detoxification
3.2.5. Novel Detoxification Strategies
Nanoparticles
Plant Extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2019, 59, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Mousavi Khaneghah, A.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Bennett, J.W. Mycotoxins, mycotoxicoses, mycotoxicology and Mycopathologia. Mycopathologia 1987, 100, 3–5. [Google Scholar] [CrossRef]
- Pittet, A.C. Natural occurrence of mycotoxins in foods and feeds: An update review. Rev. Med. Vet. 1998, 6, 479–492. [Google Scholar]
- Kagot, V.; Okoth, S.; De Boevre, M.; De Saeger, S. Biocontrol of aspergillus and fusarium mycotoxins in Africa: Benefits and limitations. Toxins 2019, 11, 109. [Google Scholar] [CrossRef] [Green Version]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Winter, G.; Pereg, L. A review on the relation between soil and mycotoxins: Effect of aflatoxin on field, food and finance. Eur. J. Soil Sci. 2019, 70, 882–897. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Ayofemi Olalekan Adeyeye, S. Aflatoxigenic fungi and mycotoxins in food: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aldars-garcía, L.; Berman, M.; Ortiz, J.; Ramos, A.J.; Marín, S. Probability models for growth and aflatoxin B1 production as affected by intraspecies variability in Aspergillus flavus. Food Microbiol. 2018, 72, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, J. Natural occurrence of mycotoxins in growing and conserved forage crops. In Mycotoxins and Animal Foods; Smith, J.E., Henderson, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 363–397. [Google Scholar]
- Rodrigues, P.; Venâncio, A.; Lima, N. Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res. Int. 2012, 48, 76–90. [Google Scholar] [CrossRef]
- Fountain, J.C.; Khera, P.; Yang, L.; Nayak, S.N.; Scully, B.T.; Lee, R.D.; Chen, Z.Y.; Kemerait, R.C.; Varshney, R.K.; Guo, B. Resistance to Aspergillus flavus in maize and peanut: Molecular biology, breeding, environmental stress, and future perspectives. Crop J. 2015, 3, 229–237. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- European Food Safety Authority. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Varzakas, T. Quality and safety aspects of cereals (Wheat) and their products. Crit. Rev. Food Sci. Nutr. 2016, 56, 2495–2510. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Kouidhi, B.; Al Qurashi, Y.M.A.; Ben Salah-Abbès, J.; Chaieb, K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon 2019, 160, 12–22. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2009_en.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2010_en.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2011_en.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2012_en.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2013.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2014.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2015.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2016.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2017.pdf (accessed on 19 October 2019).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2018.pdf (accessed on 19 October 2019).
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temba, B.A.; Fletcher, M.T.; Fox, G.P.; Harvey, J.; Okoth, S.A.; Sultanbawa, Y. Curcumin-based photosensitization inactivates Aspergillus flavus and reduces aflatoxin B1 in maize kernels. Food Microbiol. 2019, 82, 82–88. [Google Scholar] [CrossRef]
- WHO (World Health Organization). New IARC report urges action against widespread mycotoxin contamination in developing countries. IARC WHO Press Release 2016, 9, 2015–2016. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 1881/2006 of 19 December 2006. Off. J. Eur. Union 2006, 364, 5. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1126/2007, on setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, 14–17. [Google Scholar]
- Piacentini, K.C.; Ferranti, L.S.; Pinheiro, M.; Bertozzi, B.G.; Rocha, L.O. Mycotoxin contamination in cereal-based baby foods. Curr. Opin. Food Sci. 2019, 30, 73–78. [Google Scholar] [CrossRef]
- Ünüsan, N. Systematic review of mycotoxins in food and feeds in Turkey. Food Control 2019, 97, 1–14. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation No 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 91, 12–15. [Google Scholar]
- Debegnach, F.; Patriarca, S.; Brera, C.; Gregori, E.; Sonego, E.; Moracci, G.; De Santis, B. Ergot Alkaloids in Wheat and Rye Derived Products in Italy. Foods 2019, 8, 8050150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority; Arcella, D.; Eskola, M.; Gomez Ruiz, J.Á. Dietary exposure assessment to Alternaria toxins in the European population European. EFSA J. 2016, 14, 4654. [Google Scholar]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta. 2019, 1069, 1–27. [Google Scholar] [CrossRef]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009, 28, 142–153. [Google Scholar] [CrossRef]
- Reiter, E.; Zentek, J.; Razzazi, E. Review on sample preparation strategies and methods used for the analysis of aflatoxins in food and feed. Mol. Nutr. Food Res. 2009, 53, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Frisvad, J.C.; Ramson, R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Rirsks to Humans; World Health Organization: Lyon, France, 1993; Volume 56, pp. 1–609. [Google Scholar]
- Hernández-Martínez, R.; Navarro-Blasco, I. Aflatoxin levels and exposure assessment of Spanish infant cereals. Food Addit. Contam. Part B Surveill. 2010, 3, 275–288. [Google Scholar] [CrossRef]
- Zinedine, A.; Mañes, J. Occurrence and legislation of mycotoxins in food and feed from Morocco. Food Control 2009, 20, 334–344. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Agosti, B.; Donadini, G. Transfer of aflatoxin B1 and fumonisin B1 from naturally contaminated raw materials to beer during an industrial brewing process. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1431–1439. [Google Scholar] [CrossRef]
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the mycotoxins incidence in Serbia in the period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; André El Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 11060304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunarathna, N.B.; Fernando, C.J.; Munasinghe, D.M.S.; Fernando, R. Occurrence of aflatoxins in edible vegetable oils in Sri Lanka. Food Control 2019, 101, 97–103. [Google Scholar] [CrossRef]
- Blankson, G.K.; Mills-Robertson, F.C.; Ofosu, I.W. Survey of occurrence levels of Aflatoxins in selected locally processed cereal-based foods for human consumption from Ghana. Food Control 2019, 95, 170–175. [Google Scholar] [CrossRef]
- Quevedo-Garza, P.A.; Amador-Espejo, G.G.; Cantú-Martínez, P.C.; Trujillo-Mesa, J.A. Aflatoxin M1 occurrence in fluid milk commercialized in Monterrey, Mexico. J. Food Saf. 2018, 38, 1–4. [Google Scholar] [CrossRef]
- Kebede, H.; Abbas, H.K.; Fisher, D.K.; Bellaloui, N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins 2012, 4, 1385–1403. [Google Scholar] [CrossRef]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 66–72. [Google Scholar]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.-N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Gross-Steinmeyer, K.; Eaton, D.L. Dietary modulation of the biotransformation and genotoxicity of aflatoxin B1. Toxicology 2012, 299, 69–79. [Google Scholar] [CrossRef]
- Jaimez, J.; Fente, C.A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A.; Mahuzier, G.; Prognon, P. Application of the assay of aflatoxins by liquid chromatography with fluorescence detection in food analysis. J. Chromatogr. A 2000, 882, 1–10. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2009, 31, 71–82. [Google Scholar] [CrossRef]
- Okioma, M.N. 2004 and 2005 Afalatoxin tragedies in Kenya–A case study. In Mycotoxins, Detection Methods, Management, Puplic Health and Agricultural, Trade; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; Cromwell Press: London, UK, 2008; pp. 127–133. [Google Scholar]
- Camiletti, B.X.; Torrico, A.K.; Fernanda Maurino, M.; Cristos, D.; Magnoli, C.; Lucini, E.I.; Pecci, M.D.L.P.G. Fungal screening and aflatoxin production by Aspergillus section Flavi isolated from pre-harvest maize ears grown in two Argentine regions. Crop Prot. 2017, 92, 41–48. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination Across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 11070411. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Li, S.; Bai, Y.; Prates, L.L.; Lei, Y.; Yu, P. Mycotoxin contamination of food and feed in China: Occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Assunção, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate change and the health impact of aflatoxins exposure in Portugal–an overview. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1610–1621. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Manzanares, N.; Rdríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Mycotoxins in Swine Feeding from Spain. Toxins 2019, 11, 342. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Munissi, J.J.E.; Nyandoro, S.S. Aflatoxins in sunflower seeds and unrefined sunflower oils from Singida, Tanzania. Food Addit. Contam. Part B Surveill. 2018, 11, 161–166. [Google Scholar] [CrossRef]
- Kluczkovski, A.M. Fungal and mycotoxin problems in the nut industry. Curr. Opin. Food Sci. 2019, 29, 56–63. [Google Scholar] [CrossRef]
- El Darra, N.; Gambacorta, L.; Solfrizzo, M. Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control 2019, 95, 63–70. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Han, S.Y.; Li, M.; Ma, T.-Z.; Sheng, W.-J.; Zhu, X. Simultaneous analysis of 20 mycotoxins in grapes and wines from Hexi Corridor region (China): Based on a QuEChERS–UHPLC–MS/MS method. Molecules 2018, 23, 22981926. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.J.; Iamanaka, B.T.; Fungaro, M.H.P.; Taniwaki, M.H. Aflatoxins in sugarcane production chain: What could be the source? Curr. Opin. Food Sci. 2019, 29, 94–98. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Krska, R.; Sulyok, M. Occurrence of Ochratoxins, Fumonisin B2, Aflatoxins (B1 and B2), and Other Secondary Fungal Metabolites in Dried Date Palm Fruits from Egypt: A Mini-Survey. J. Food Sci. 2018, 83, 559–564. [Google Scholar] [CrossRef]
- Akbar, N.; Nasir, M.; Naeem, N.; Ahmad, M.-U.-D.; Iqbal, S.; Rashid, A.; Imran, M.; Gondal, T.A.; Atif, M.; Salehi, B.; et al. Occurrence and Seasonal Variations of Aflatoxin M1 in Milk from Punjab, Pakistan. Toxins 2019, 11, 11100574. [Google Scholar] [CrossRef] [Green Version]
- Fouad, A.M.; Ruan, D.; El Senouse, H.A.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin B1 produced by aspergillus flavus and aspergillus parasiticus strains on poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Perrone, G.; Rodriguez, A.; Magistà, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27. [Google Scholar] [CrossRef]
- Khanian, M.; Karimi-Torshizi, M.A.; Allameh, A. Alleviation of aflatoxin-related oxidative damage to liver and improvement of growth performance in broiler chickens consumed Lactobacillus plantarum 299v for entire growth period. Toxicon 2019, 158, 57–62. [Google Scholar] [CrossRef]
- Guo, L.Y.; Zheng, N.; Zhang, Y.D.; Du, R.H.; Zheng, B.Q.; Wang, J.Q. A survey of seasonal variations of aflatoxin M1 in raw milk in Tangshan region of China during 2012–2014. Food Control 2016, 69, 30–35. [Google Scholar] [CrossRef]
- Anthony, M.H.; Ojochenemi, A.D.; Mulunda, M.; Oriyomi, S.T.; Jideofor, N.F.; Tunde, O.; Seun, E.O.; Umuhani, Y.O.; Robertson, O.B.; Isah, A.; et al. Aflatoxin M1 in Breast Milk, Cow Milk and Milk Products in Minna, Nigeria and their Predisposing Factors. Biochem. Anal. Biochem. 2016, 5, 4. [Google Scholar] [CrossRef]
- Ware, L.Y.; Durand, N.; Nikiema, P.A.; Alter, P.; Fontana, A.; Montet, D.; Barro, N. Occurrence of mycotoxins in commercial infant formulas locally produced in Ouagadougou (Burkina Faso). Food Control 2017, 73, 518–523. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Survey of 11 mycotoxins in wheat flour in Hebei province, China. Food Addit. Contam. Part B Surveill. 2015, 8, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2014, 50, 401–404. [Google Scholar] [CrossRef]
- Xing, F.; Liu, X.; Wang, L.; Selvaraj, J.N.; Jin, N.; Wang, Y.; Zhao, Y.; Liu, Y. Distribution and variation of fungi and major mycotoxins in pre- and post-nature drying maize in North China Plain. Food Control 2017, 80, 244–251. [Google Scholar] [CrossRef]
- Golge, O.; Hepsag, F.; Kabak, B. Determination of aflatoxins in walnut sujuk and Turkish delight by HPLC-FLD method. Food Control 2016, 59, 731–736. [Google Scholar] [CrossRef]
- Yilmaz, S.Ö. The contamination rate of aflatoxins in ground red peppers, dried figs, walnuts without shell and seedless black raisins commercialized in Sakarya City Center, Turkey. Ital. J. Food Sci. 2017, 29, 591–598. [Google Scholar]
- Lippolis, V.; Irurhe, O.; Porricelli, A.C.R.; Cortese, M.; Schena, R.; Imafidon, T.; Oluwadun, A.; Pascale, M. Natural co-occurrence of aflatoxins and ochratoxin A in ginger (Zingiber officinale) from Nigeria. Food Control 2017, 73, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Amri, E. Aflatoxin and Fumonisin Contamination of Sun-Dried Sweet Potato (Ipomoea batatas L.) Chips in Kahama District, Tanzania. J. Appl. Environ. Microbiol. 2016, 4, 55–62. [Google Scholar]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Alhamoud, Y.; Yang, D.; Kenston, S.S.F.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhaoa, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef]
- Temesgen, A.; Teshome, G. Major mycotoxins occurrence, prevention and control approaches. Biotechnol. Mol. Biol. Rev. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Miller, J.D.; Schaafsma, A.W. Occurrence of Penicillium verrucosum, ochratoxin A, ochratoxin B and citrinin in on-farm stored winter wheat from the Canadian Great Lakes Region. PLoS ONE 2017, 12, e0181239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, Y.-C.; Bai, X.-L.; Liu, Y.-M.; Wu, G.-F.; Yang, F.-S.; Liao, X. Multi-mycotoxins analysis in liquid milk by UHPLC-Q-Exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes. Food Chem. 2020, 305, 125429. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Joint Food and Agriculture Organization; World Health Organization Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants: Fifty-Fifth Report of the JOINT/FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2001; p. 701. [Google Scholar]
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request From the Commission Related To Ochratoxin a in Food. EFSA J. 2006, 365, 1–56. [Google Scholar]
- Arrúa, A.A.; Mendes, J.M.; Arrúa, P.; Ferreira, F.P.; Caballero, G.; Cazal, C.; Kohli, M.M.; Peralta, I.; Ulke, G.; Ríos, D.F. Occurrence of Deoxynivalenol and Ochratoxin A in Beers and Wines Commercialized in Paraguay. Toxins 2019, 11, 308. [Google Scholar] [CrossRef] [Green Version]
- Balendres, M.A.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic Fungi and Mycotoxins in Agricultural Crop Commodities in the Philippines: A Review. Foods 2019, 8, 8070249. [Google Scholar] [CrossRef] [Green Version]
- Kedjebo, K.B.D.; Guehi, T.S.; Kouakou, B.; Durand, N.; Aguilar, P.; Fontana, A.; Montet, D. Effect of post-harvest treatments on the occurrence of ochratoxin a in raw cocoa beans. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 33, 157–166. [Google Scholar] [CrossRef]
- Naz, N.; Kashif, A.; Kanwal, K.; Ajaz, H. Incidence of mycotoxins in local and branded samples of chocolates marketed in Pakistan. J. Food Qual. 2017, 2017, 1947871. [Google Scholar] [CrossRef] [Green Version]
- Meftah, S.; Abid, S.; Dias, T.; Rodrigues, P. Mechanisms underlying the effect of commercial starter cultures and a native yeast on ochratoxin A production in meat products. LWT 2020, 117, 108611. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–17. [Google Scholar] [CrossRef]
- Duarte, S.C.; Pena, A.; Lino, C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Zong, N.; Zhou, Z.; Ma, L. Ochratoxin A in dried vine fruits from Chinese markets. Food Addit. Contam. Part B Surveill. 2014, 7, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, Y.; Jiang, D.; Feng, X.; Li, J.; Wang, M. Survey of alternaria toxins and other mycotoxins in dried fruits in China. Toxins 2017, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darouj, E.; Massouh, L.; Ghanem, I. Investigation of ochratoxin A in Syrian consumed baby foods. Food Control 2016, 62, 97–103. [Google Scholar] [CrossRef]
- Tonon, K.M.; Reiter, M.G.R.; Savi, G.D.; Scussel, V.M. Human milk AFM1, OTA, and DON evaluation by liquid chromatography tandem mass specrometry and their relation to the Southern Brazil nursing mothers’ diet. J. Food Saf. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Çağindi, Ö.; Gürhayta, O.F. Aflatoxins and ochratoxin A in dried eggplant and green bell pepper. Food Control 2016, 70, 216–220. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2017, 97, 1805–1810. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Al Thani, R.A.; Atia, F.; Al Meer, S.; Migheli, Q.; Jaoua, S. Co-occurrence of mycotoxins in commercial formula milk and cereal-based baby food on the Qatar market. Food Addit. Contam. Part B Surveill. 2018, 11, 191–197. [Google Scholar] [CrossRef]
- Gherbawy, Y.; Shebany, Y. Mycobiota, total aflatoxins and ochratoxin a of cardamom pods. Food Sci. Technol. Res. 2018, 24, 87–96. [Google Scholar] [CrossRef]
- Benites, A.J.; Fernandes, M.; Boleto, A.R.; Azevedo, S.; Silva, S.; Leitão, A.L. Occurrence of ochratoxin A in roasted coffee samples commercialized in Portugal. Food Control 2017, 73, 1223–1228. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Ngemela, A.F.; Jensen, L.B.; De Medeiros, L.S.; Rasmussen, P.H. UHPLC-MS/MS determination of ochratoxin a and fumonisins in coffee using QuEChERS extraction combined with mixed-mode SPE purification. J. Agric. Food Chem. 2015, 63, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordind, K.; Gomese, G.A.; Alvito, P.; Assunção, R.; Oliveira, C.A.F. Assessment of mycotoxin exposure and risk characterization using occurrence data in foods and urinary biomarkers in Brazil. Food Chem. Toxicol. 2019, 128, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; Eyangoh, S.; Verger, P.; et al. Regional sub-saharan Africa total diet study in benin, cameroon, mali and nigeria reveals the presence of 164 mycotoxins and other secondary metabolites in foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [Green Version]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Perši, N.; Pleadin, J.; Kovačević, D.; Scortichini, G.; Milone, S. Ochratoxin A in raw materials and cooked meat products made from OTA-treated pigs. Meat Sci. 2014, 96, 203–210. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, J.M.; Frisvad, J.C.; Thrane, U.; Nielsen, K.F. Production of fumonisin B2 and B4 by aspergillus niger on grapes and raisins. J. Agric. Food Chem. 2010, 58, 954–958. [Google Scholar] [CrossRef]
- Alberts, J.F.; van Zyl, W.H.; Gelderblom, W.C.A. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Front. Microbiol. 2016, 7, 201600548. [Google Scholar] [CrossRef] [Green Version]
- Joint Food and Agriculture Organization; World Health Organization Expert Committee on Food Additives (JECFA). Co-Exposure of Fumonisins with Aflatoxins; Food Safety Digest; World Health Organization: Geneva, Switzerland, 2018; pp. 1–4. [Google Scholar]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; van der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 1988, 55, 197–203. [Google Scholar]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Safety evaluation of certain mycotoxins in food (WHO food additives series 47). In International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 2001; pp. 103–279. [Google Scholar]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, W.; Li, H.; Iqbal, J.; Zhu, Y.; Wu, T.; Du, Y. Rapid determination of fumonisin (FB1) by syringe SPE coupled with solid-phase fluorescence spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117549. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Nichea, M.J.; Monge, M.P.; Sulyok, M.; Chiacchiera, S.M.; Ramirez, M.L. Fumonisin occurrence in wheat-based products from Argentina. Food Addit. Contam. Part B Surveill. 2019, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Chiotta, M.L.; Zachetti, V.; Chulze, S.N.; Ramirez, M.L. Fumonisins and fumonisin-producing Fusarium occurrence in wheat and wheat by products: A review. J. Cereal Sci. 2018, 80, 158–166. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Battilani, P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016, 9, 727–739. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A.; Urbaniak, M. Wildly Growing Asparagus (Asparagus officinalis L.) Hosts Pathogenic Fusarium Species and Accumulates Their Mycotoxins. Microb. Ecol. 2016, 71, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Al-Taher, F.; Cappozzo, J.; Zweigenbaum, J.; Lee, H.J.; Jackson, L.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the U.S. market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Kos, J.; Hajnal, E.J.; Škrinjar, M.; Mišan, A.; Mandić, A.; Jovanov, P.; Milovanović, I. Presence of Fusarium toxins in maize from Autonomous Province of Vojvodina, Serbia. Food Control 2014, 46, 98–101. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Zheng, F.; Chen, J.; Li, W. Fumonisins B1, B2 and B3 in corn products, wheat flour and corn oil marketed in Shandong province of China. Food Addit. Contam. Part B Surveill. 2015, 8, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.D.R.L.; Reis, T.A.D.; Corrêa, B.; Badiale-Furlong, E. Mycobiota and occurrence of Fumonisin B1 in wheat harvested in Southern Brazil. Ciênc. Rural 2015, 45, 1050–1057. [Google Scholar] [CrossRef]
- Amato, B.; Pfohl, K.; Tonti, S.; Nipoti, P.; Dastjerdi, R.; Pisi, A.; Karlovsky, P.; Prodi, A. Fusarium proliferatum and fumonisin B1 co-occur with Fusarium species causing Fusarium Head Blight in durum wheat in Italy. J. Appl. Bot. Food Qual. 2015, 88, 228–233. [Google Scholar]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC-MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 21050627. [Google Scholar] [CrossRef] [Green Version]
- Krska, R.; Malachova, A.; Berthiller, F.; van Egmond, H.P. Determination of T-2 and HT-2 toxins in food and feed: An update. World Mycotoxin J. 2014, 7, 131–142. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliott, C.T.; Campbell, K. Current trends in rapid tests for mycotoxins. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 800–814. [Google Scholar] [CrossRef] [Green Version]
- Pascari, X.; Marín, S.; Ramos, A.J.; Molino, F.; Sanchis, V. Deoxynivalenol in cereal-based baby food production process. A review. Food Control 2019, 99, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, A.; Horsley, R.; Jack, M.M.; Kobielush, B.; Ryu, D.; Tittlemier, S.; Wilson, W.W.; Abbas, H.K.; Abel, S.; Harrison, G.; et al. DON Occurrence in Grains: A North American Perspective. Cereal Foods World 2015, 60. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Rath, S.K. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Joint Food and Agriculture Organization; World Health Organization Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants (Seventy-third Report of the Joint FAO/WHO Expert Committee on Food Current Additives); WHO: Geneva, Switzerland, 2010; p. 227. [Google Scholar]
- European Food Safety Authority. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of Fusarium mycotoxins in cereal crops and processed products (Ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oueslati, S.; Berrada, H.; Mañes, J.; Juan, C. Presence of mycotoxins in Tunisian infant foods samples and subsequent risk assessment. Food Control 2018, 84, 362–369. [Google Scholar] [CrossRef]
- Pleadin, J.; Staver, M.M.; Markov, K.; Frece, J.; Zadravec, M.; Jaki, V.; Krupić, I.; Vahčić, N. Mycotoxins in organic and conventional cereals and cereal products grown and marketed in Croatia. Mycotoxin Res. 2017, 33, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Levasseur-Garcia, C. Updated overview of infrared spectroscopy methods for detecting mycotoxins on cereals (corn, wheat, and barley). Toxins 2018, 10, 10010038. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.W.; De Saeger, S.; Wu, A. Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: Case study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef]
- Urusov, A.E.; Gubaidullina, M.K.; Petrakova, A.V.; Zherdev, A.V.; Dzantiev, B.B. A new kind of highly sensitive competitive lateral flow immunoassay displaying direct analyte-signal dependence. Application to the determination of the mycotoxin deoxynivalenol. Microchim. Acta 2018, 185, 29. [Google Scholar] [CrossRef]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Habib, F.-E.; Rahman, M.; Iqbal, M. Multiple mycotoxins in rice: Occurrence and health risk assessment in children and adults of Punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima Rocha, D.F.; dos Santos Oliveira, M.; Furlong, E.B.; Junges, A.; Paroul, N.; Valduga, E.; Toniazzo, G.B.; Zeni, J.; Cansian, R.L. Evaluation of the TLC quantification method and occurrence of deoxynivalenol in wheat flour of southern Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, T.; Leggieri, M.C.; Battilani, P.; Pietri, A. Co-occurrence of type A and B trichothecenes and zearalenone in wheat grown in northern Italy over the years 2009–2011. Food Addit. Contam. Part B Surveill. 2014, 7, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Tibola, C.S.; Scussel, V.M. Mycoflora and deoxynivalenol in whole wheat grains (Triticum aestivum L.) from Southern. Food Addit. Contam. Part B. 2014, 7, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Darsanaki, R.K.; Issazadeh, K.; Aliabadi, M.A.; Chakoosari, M.M.D. Occurrence of Deoxynivalenol (DON) in wheat flours in Guilan Province, northern Iran. Ann. Agric. Environ. Med. 2015, 22, 35–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, A.P.; Lamardo, L.C.A.; Shundo, L.; da Silva, S.A.; Navas, S.A.; Alaburda, J.; Ruvieri, V.; Sabino, M. Occurrence of deoxynivalenol in wheat flour, instant noodle and biscuits commercialised in Brazil. Food Addit. Contam. Part B Surveill. 2016, 9, 251–255. [Google Scholar] [CrossRef]
- Calori-Domingues, M.A.; Bernardi, C.M.G.; Nardin, M.S.; de Souza, G.V.; dos Santos, F.G.R.; de Abreu Stein, M.; da Gloria, E.M.; dos Santos Dias, C.T.; de Camargo, A.C. Co-occurrence and distribution of deoxynivalenol, nivalenol and zearalenone in wheat from Brazil. Food Addit. Contam. Part B Surveill. 2016, 9, 142–151. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; De Souza Garcia, F.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef]
- Tima, H.; Brückner, A.; Mohácsi-Farkas, C.; Kiskó, G. Fusarium mycotoxins in cereals harvested from Hungarian fields. Food Addit. Contam. Part B Surveill. 2016, 9, 127–131. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C.; Torres, A.M. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium mycotoxins in Swiss wheat: A survey of growers’ samples between 2007 and 2014 shows strong year and minor geographic effects. Toxins 2017, 9, 9080246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.V.; Pante, G.C.; Romoli, J.C.Z.; de Souza, A.P.M.; da Rocha, G.H.O.; Ferreira, F.D.; Feijó, A.L.R.; Moscardi, S.M.P.; de Paula, K.R.; Bando, E.; et al. Occurrence and risk assessment of population exposed to deoxynivalenol in foods derived from wheat flour in Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in polish winter wheat. Toxins 2018, 10, 10020081. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Guan, X.; Zong, Y.; Hua, X.; Xing, F.; Wang, Y.; Wang, F.; Liu, Y. Deoxynivalenol in wheat from the Northwestern region in China. Food Addit. Contam. Part B Surveill. 2018, 11, 281–285. [Google Scholar] [CrossRef]
- Torović, L. Fusarium toxins in corn food products: A survey of the Serbian retail market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1596–1609. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; Almeida, F.G.; Minella, E.; Corrêa, B. Occurrence of deoxynivalenol and zearalenone in brewing barley grains from Brazil. Mycotoxin Res. 2018, 34, 173–178. [Google Scholar] [CrossRef]
- Gummadidala, P.M.; Omebeyinje, M.H.; Burch, J.A.; Chakraborty, P.; Biswas, P.K.; Banerjee, K.; Wang, Q.; Jesmin, R.; Mitra, C.; Moeller, P.D.R.; et al. Complementary feeding may pose a risk of simultaneous exposures to aflatoxin M1 and deoxynivalenol in Indian infants and toddlers: Lessons from a mini-survey of food samples obtained from Kolkata, India. Food Chem. Toxicol. 2019, 123, 9–15. [Google Scholar] [CrossRef]
- Edwards, S.G. Impact of agronomic and climatic factors on the mycotoxin content of harvested oats in the United Kingdom. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 2230–2241. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 8060160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elaridi, J.; Yamani, O.; Al Matari, A.; Dakroub, S.; Attieh, Z. Determination of Ochratoxin A (OTA), Ochratoxin B (OTB), T-2, and HT-2 Toxins in Wheat Grains, Wheat Flour, and Bread in Lebanon by LC-MS/MS. Toxins 2019, 11, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Sancho, D.G.; Valle-Algarra, F.M.; Jiménez, M.; Burdaspal, P.; Legarda, T.M.; Ramos, A.J.; Sanchis, V.; Marín, S. Presence of trichothecenes and co-occurrence in cereal-based food from Catalonia (Spain). Food Control 2011, 22, 490–495. [Google Scholar] [CrossRef]
- Rodrνguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 28–40. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–20. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the risks for human and animal health related to the presence of modeified forms of certain mycotoxins in food and feed. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Habler, K.; Gotthardt, M.; Schüler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC—MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Tamura, M.; Mochizuki, N.; Nagatomi, Y.; Harayama, K.; Toriba, A.; Hayakawa, K. A Method for Simultaneous Determination of 20 Fusarium Toxins in Cereals by High-Resolution Liquid Chromatography-Orbitrap Mass Spectrometry with a Pentafluorophenyl Column. Toxins 2015, 7, 1664–1682. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Girgin, G.; Baydar, T. Mycotoxin Detection in Maize, Commercial Feed, and Raw Dairy Milk Samples from Assiut City, Egypt. Vet. Sci. 2019, 6, 6020057. [Google Scholar] [CrossRef] [Green Version]
- Tulayakul, P.; Sugita-konishi, Y. Mycotoxin contamination in foodstuffs and feeds- health concerns in Thailand. Jpn. J. Vet. Res. 2017, 65, 173–183. [Google Scholar]
- Nazari, F.; Sulyok, M.; Yazdanpanah, H.; Kobarfard, F.; Krska, R. A survey of mycotoxins in domestic rice in Iran by liquid chromatography tandem mass spectrometry. Toxicol. Mech. Method 2014, 1, 37–41. [Google Scholar] [CrossRef]
- Morton, I.K.; Hall, J.M. Concise Dictionary of Pharmacological Agents: Properties and Synonyms; Springer Science & Business Media: Berlin, Germany, 2012; p. 295. [Google Scholar]
- Han, Z.; Jiang, K.; Fan, Z.; Di Mavungu, J.D.; Dong, M.; Guo, W.; Fan, K.; Campbell, K.; Zhao, Z.; Wu, Y. Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control 2017, 79, 177–184. [Google Scholar] [CrossRef]
- Ok, H.E.; Choi, S.W.; Kim, M.; Chun, H.S. HPLC and UPLC methods for the determination of zearalenone in noodles, cereal snacks and infant formula. Food Chem. 2014, 163, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lanza, A.; da Silva, R.C.; dos Santos, I.D.; Pizzutti, I.R.; Cence, K.; Cansian, R.L.; Zeni, J.; Valduga, E. Mycotoxins’ evaluation in wheat flours used in Brazilian bakeries. Food Sci. Biotechnol. 2019, 28, 931–937. [Google Scholar] [CrossRef]
- Vaclavikova, M.; Malachova, A.; Veprikova, Z.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. “Emerging” mycotoxins in cereals processing chains: Changes of enniatins during beer and bread making. Food Chem. 2013, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging fusarium and alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Luz, C.; Saladino, F.; Luciano, F.B.; Mañes, J.; Meca, G. Occurrence, toxicity, bioaccessibility and mitigation strategies of beauvericin, a minor Fusarium mycotoxin. Food Chem. Toxicol. 2017, 107, 430–439. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Presence of enniatins and beauvericin in Romanian wheat samples: From raw material to products for direct human consumption. Toxins 2017, 9, 9060189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiles, J.M.; Saladino, F.; Mañes, J.; Fernández-Franzón, M.; Meca, G. Occurrence of mycotoxins in refrigerated pizza dough and risk assessment of exposure for the Spanish population. Food Chem. Toxicol. 2016, 94, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Suzuki, Y.; Sugita-Konishi, Y.; Ohnishi, T.; Terajima, J. Occurrence of beauvericin and enniatins in wheat flour and corn grits on the Japanese market, and their co-contamination with type B trichothecene mycotoxins. Food Addit. Contam. Part A Chem. Anal. Control.Expo. Risk Assess. 2016, 33, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Beed, F.; Boni, S.; Abass, A.; Mukunzi, A.; Krska, R. Quantitation of multiple mycotoxins and cyanogenic glucosides in cassava samples from Tanzania and Rwanda by an LC-MS/MS-based multi-toxin method. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Sulyok, M.; Kobarfard, F.; Yazdanpanah, H.; Krska, R. Evaluation of Emerging Fusarium mycotoxins Beauvericin, Enniatins, Fusaproliferin and Moniliformin in Domestic Rice in Iran. Iran. J. Pharm. Res. 2013, 14, 505–512. [Google Scholar]
- Blesa, J.; Moltó, J.C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene Mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 201900403. [Google Scholar] [CrossRef]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Agriopoulou, S. Enniatins: An Emerging Food Safety Issue. EC Nutr. 2016, 3, 1142–1146. [Google Scholar]
- Gunter, A.B.; Hermans, A.; Bosnich, W.; Johnson, D.A.; Harris, L.J.; Gleddie, S. Protein engineering of Saccharomyces cerevisiae transporter Pdr5p identifies key residues that impact Fusarium mycotoxin export and resistance to inhibition. Microbiologyopen 2016, 5, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Meca, G.; Juan, C.; Mañes, J. Biosynthesis of beauvericin and enniatins in vitro by wheat Fusarium species and natural grain contamination in an area of central Italy. Food Microbiol. 2015, 46, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Jajic, I.; Dudaš, T.; Krstovic, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savic, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 357, 1–14. [Google Scholar]
- European Food Safety Authority. Scientific report on human and animal dietary exposure to ergot alkaloids. EFSA J. 2017, 15, 4902. [Google Scholar]
- Shi, H.; Schwab, W.; Liu, N.; Yu, P. Major ergot alkaloids in naturally contaminated cool-season barley grain grown under a cold climate condition in western Canada, explored with near-infrared (NIR) and fourier transform mid-infrared (ATR-FT/MIR) spectroscopy. Food Control 2019, 102, 221–230. [Google Scholar] [CrossRef]
- European Food Safety Authority. Panel on Contaminants in the Food Chain (CONTAM); Scientific O pinion on Ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798. [Google Scholar]
- Topi, D.; Jakovac-Strajn, B.; Pavšič-Vrtač, K.; Tavčar-Kalcher, G. Occurrence of ergot alkaloids in wheat from Albania. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1333–1343. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; McKendry, T. Occurrence of Ergot and Ergot Alkaloids in Western Canadian Wheat and Other Cereals. J. Agric. Food Chem. 2015, 63, 6644–6650. [Google Scholar] [CrossRef]
- Commission Recommendation, 2012/154/EU on the monitoring of the presence of ergot alkaloids in feed and food. Off. J. Eur. Union 2012, L77, 20–21.
- Guo, Q.; Shao, B.; Du, Z.; Zhang, J. Simultaneous Determination of 25 Ergot Alkaloids in Cereal Samples by Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 7033–7039. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Sivagnanam, K.; Komatsu, E.; Rampitsch, C.; Perreault, H.; Gräfenhan, T. Rapid screening of Alternaria mycotoxins using MALDI-TOF mass spectrometry. J. Sci. Food Agric. 2017, 97, 357–361. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 10, 2407. [Google Scholar]
- Zwickel, T.; Klaffke, H.; Richards, K.; Rychlik, M. Development of a high performance liquid chromatography tandem mass spectrometry based analysis for the simultaneous quantification of various Alternaria toxins in wine, vegetable juices and fruit juices. J. Chromatogr. A 2016, 1455, 74–85. [Google Scholar] [CrossRef]
- Romero Bernal, Á.R.; Reynoso, C.M.; García Londoño, V.A.; Broggi, L.E.; Resnik, S.L. Alternaria toxins in Argentinean wheat, bran, and flour. Food Addit. Contam. Part B Surveill. 2019, 12, 24–30. [Google Scholar] [CrossRef]
- Pero, R.W.; Posner, H.; Blois, M.; Harvan, D.; Spalding, J.W. Toxicity of metabolites produced by the “Alternaria”. Environ. Health Perspect. 1973, 4, 87–94. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Gaba, D.; Mckendry, T.; Pleskach, K.; Roscoe, M. Fusarium and Alternaria mycotoxins present in Canadian wheat and durum harvest samples. Can. J. Plant Pathol. 2019, 41, 403–414. [Google Scholar] [CrossRef]
- López, P.; Venema, D.; Mol, H.; Spanjer, M.; de Stoppelaar, J.; Pfeiffer, E.; de Nijs, M. Alternaria toxins and conjugates in selected foods in the Netherlands. Food Control 2016, 69, 153–159. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Gallone, T.; Garganese, F.; Caruso, A.G.; Amenduni, M.; Ippolito, A. Contamination of fresh and dried tomato by Alternaria toxins in southern Italy. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 789–799. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural occurrence of Alternaria toxins in the 2015 wheat from anhui province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Shao, B.; Yang, D.; Li, F.; Zhu, J. Natural occurrence of Alternaria toxins in wheat-based products and their dietary exposure in China. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambacorta, L.; Magistá, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018, 11, 159–173. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. A new method for detection of five alternaria toxins in food matrices based on LC-APCI-MS. Food Chem. 2013, 140, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Walravens, J.; Mikula, H.; Rychlik, M.; Asamd, S.; Ediagea, E.N.; Di Mavungua, J.D.; Landschoote, A.V.; Vanhaeckef, L.; De Saeger, S. Development and validation of an ultra-high-performance liquid chromatography tandem mass spectrometric method for the simultaneous determination of free and conjugated Alternaria toxins in cereal-based foodstuffs. J. Chromatogr. A 2014, 1372, 91–101. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in apples and apple-based food products: The burdens and the mitigation strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Patras, A.; Pokharel, B.; Bansode, R.R.; Begum, A.; Sasges, M. Patulin degradation and cytotoxicity evaluation of UV irradiated apple juice using human peripheral blood mononuclear cells. J. Food Process Eng. 2017, 40, 1–9. [Google Scholar] [CrossRef]
- Torović, L.; Dimitrov, N.; Assunção, R.; Alvito, P. Risk assessment of patulin intake through apple-based food by infants and preschool children in Serbia. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 2023–2032. [Google Scholar] [CrossRef]
- Wei, D.-M.; Xu, J.; Dong, F.-S.; Liu, X.-G.; Wu, X.-H.; Zheng, Y.-Q. Penicillium and patulin distribution in pears contaminated with Penicillium expansum. Determination of patulin in pears by UHPLC-MS/MS. J. Integr. Agric. 2017, 16, 1645–1651. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Ji, X.; Li, R.; Yang, H.; Qi, P.; Xiao, Y.; Qian, M. Occurrence of patulin in various fruit products and dietary exposure assessment for consumers in China. Food Control 2017, 78, 100–107. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, H.; Li, C.; Zhang, X. Ochratoxin A is degraded by Yarrowia lipolytica and generates non-toxic degradation products. World Mycotoxin J. 2016, 9, 269–278. [Google Scholar] [CrossRef]
- Hammami, W.; Al Thani, R.; Fiori, S.; Al-Meer, S.; Atia, F.A.; Rabah, D.; Migheli, Q.; Jaoua, S. Patulin and patulin producing Penicillium spp. Occurrence in apples and apple-based products including baby food. J. Infect. Dev. Ctries. 2017, 11, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouaoui, N.; Sbaii, N.; Bacha, H.; Abid-Essefi, S. Occurrence of patulin in various fruit juice marketed in Tunisia. Food Control 2015, 51, 356–360. [Google Scholar] [CrossRef]
- Torović, L.; Dimitrov, N.; Lopes, A.; Martins, C.; Alvito, P.; Assunção, R. Patulin in fruit juices: Occurrence, bioaccessibility, and risk assessment for Serbian population. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 985–995. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Khaneghah, A.M.; Campagnoll, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT Food Sci. Technol. 2017, 80, 200–207. [Google Scholar] [CrossRef]
- Lee, T.P.; Sakai, R.; Manaf, N.A.; Rodhi, A.M.; Saad, B. High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control 2014, 38, 142–149. [Google Scholar] [CrossRef]
- Juan, C.; Raiola, A.; Mañes, J.; Ritieni, A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Kayitesi, E.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Food fermentation and mycotoxin detoxification: An African perspective. Food Control 2019, 106, 106731. [Google Scholar] [CrossRef]
- Alberts, J.F.; Lilly, M.; Rheeder, J.P.; Burger, H.M.; Shephard, G.S.; Gelderblom, W.C.A. Technological and community-based methods to reduce mycotoxin exposure. Food Control 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Sarrocco, S.; Mauro, A.; Battilani, P. Use of Competitive Filamentous Fungi as anAlternative Approach for Mycotoxin Risk Reductionin Staple Cereals: State of Art and Future Perspectives. Toxins 2019, 11, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, J.; Liu, Z.; Shi, Y.; Liu, J.; Xu, X.; Hao, S.; Mu, P.; Deng, F.; Deng, Y. Aflatoxin B1 degradation and detoxification by Escherichia coli CG1061 isolated from chicken cecum. Front. Pharmacol. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. The status of fusarium mycotoxins in sub-Saharan Africa: A review of emerging trends and post-harvest mitigation strategies towards food control. Toxins 2017, 9, 9010019. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.L.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, P. Pre- and Postharvest Strategies to Minimize Mycotoxin Contamination in the Rice Food Chain. Compr. Rev. Food Sci. Food Saf. 2019, 18, 441–454. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Kamasani, J.R.; Mudili, V. Assessment of detoxification efficacy of irradiation on zearalenone mycotoxin in various fruit juices by response surface methodology and elucidation of its in-vitro toxicity. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin decontamination of food: Cold atmospheric pressure plasma versus “classic” decontamination. Toxins 2017, 9, 9050151. [Google Scholar] [CrossRef] [PubMed]
- Wielogorska, E.; Ahmed, Y.; Meneely, J.; Graham, W.G.; Elliott, C.T.; Gilmore, B.F. A holistic study to understand the detoxification of mycotoxins in maize and impact on its molecular integrity using cold atmospheric plasma treatment. Food Chem. 2019, 301, 125281. [Google Scholar] [CrossRef] [PubMed]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.-H.; Holzhauser, T.; Knorr, D.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nutr. Food Res. 2013, 57, 920–927. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Tavčar-Kalcher, G.; Babič, J.; Walsh, J.L.; Cvelbar, U. Mycotoxin Decontamination Efficacy of Atmospheric Pressure Air Plasma. Τoxins 2019, 11, 11040219. [Google Scholar] [CrossRef] [Green Version]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- González Pereyra, M.L.; Martínez, M.P.; Cavaglieri, L.R. Presence of aiiA homologue genes encoding for N-Acyl homoserine lactone-degrading enzyme in aflatoxin B1-decontaminating Bacillus strains with potential use as feed additives. Food Chem. Toxicol. 2019, 124, 316–323. [Google Scholar] [CrossRef]
- Ji, J.; Xie, W. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils. J. Hazard. Mater. 2020, 381, 120915. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involve in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Zachetti, V.G.L.; Cendoya, E.; Nichea, M.J.; Chulze, S.N.; Ramirez, M.L. Preliminary study on the use of chitosan as an eco-friendly alternative to control Fusarium growth and mycotoxin production on maize and wheat. Pathogens 2019, 8, 8010029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduceFusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Messia, M.C.; Marconi, E.; Falasca, L.; Zivoli, R.; Gambacorta, L.; Perrone, G.; Solfrizzo, M. Effect of gaseous ozone treatments on DON, microbial contaminants and technological parameters of wheat and semolina. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Porto, Y.D.; Trombete, F.M.; Freitas-Silva, O.; de Castro, I.M.; Direito, G.M.; Ascheri, J.L.R. Gaseous Ozonation to Reduce Aflatoxins Levels and Microbial Contamination in Corn Grits. Microorganisms 2019, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, A.P.S.; Castanha, N.; Calori-Domingues, M.A.; Augusto, P.E.D. Ozonation of whole wheat flour and wet milling effluent: Degradation of deoxynivalenol (DON) and rheological properties. J. Environ. Sci. Heal Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 516–524. [Google Scholar] [CrossRef]
- Alexandre, A.P.S.; Castanha, N.; Costa, N.S.; Santos, A.S.; Badiale-Furlong, E.; Augusto, P.E.D.; Calori-Dominguesa, M.A. Ozone technology to reduce zearalenone contamination in whole maize flour: Degradation kinetics and impact on quality. J. Sci. Food Agric. 2019, 99, 6814–6821. [Google Scholar] [CrossRef]
- Santos Alexandre, A.P.; Vela-Paredes, R.S.; Santos, A.S.; Costa, N.S.; Canniatti-Brazaca, S.G.; Calori-Domingues, M.A.; Augusto, P.E.D. Ozone treatment to reduce deoxynivalenol (DON) and zearalenone (ZEN) contamination in wheat bran and its impact on nutritional quality. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1189–1199. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of ozone treatment on deoxynivalenol and quality evaluation of ozonised wheat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 544–553. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226. [Google Scholar] [CrossRef]
- Li, M.; Guan, E.; Bian, K. Structure Elucidation and Toxicity Analysis of the Degradation Products of Deoxynivalenol by Gaseous Ozone. Toxins 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhu, K.X.; Wang, B.W.; Guo, X.N.; Peng, W.; Zhou, H.M. Evaluation the quality characteristics of wheat flour and shelf-life of fresh noodles as affected by ozone treatment. Food Chem. 2012, 135, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Zhou, T. Addressing the mycotoxin deoxynivalenol contamination with soil-derived bacterial and enzymatic transformations targeting the C3 carbon. World Mycotoxin J. 2018, 11, 101–111. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Li, M.; Garba, B.; Zhang, Q.; Wang, Y.; Zhang, H.; Li, P. Isolation and characterization of a Bacillus subtilis strain with aflatoxin B1 biodegradation capability. Food Control 2017, 75, 92–98. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B.; Shivakumara, P.; Kumar, J.S.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Kayitesi, E.; Njobeh, P.B. Reduction of Mycotoxins during fermentation of Whole Grain Sorghum to Whole Grain ting (A Southern African Food). Toxins 2019, 11, 11030180. [Google Scholar] [CrossRef] [Green Version]
- Tilocca, B.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. A proteomic investigation of Aspergillus carbonarius exposed to yeast volatilome or to its major component 2-phenylethanol reveals major shifts in fungal metabolism. Int. J. Food Microbiol. 2019, 306, 108265. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of yeast volatile organic compounds on ochratoxin A-producing Aspergillus carbonarius and A. ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, J.; Wang, P.; Yin, Q.; Huang, W.; Liu, C.; Bai, X.; Zhu, Q.; Gao, T.; Zhou, P. Effects of Saccharomyces cerevisiae on alleviating cytotoxicity of porcine jejunal epithelia cells induced by deoxynivalenol. AMB Express 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Mendieta, C.R.; Gomez, G.V.; Del Río, J.C.G.; Cuevas, A.C.; Arce, J.M.; Ávila, E.G. Effect of the addition of Saccharomyces Cerevisiae yeast cell walls to diets with mycotoxins on the performance and immune responses of broilers. J. Poult. Sci. 2018, 55, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, M.; Wu, C.; Peng, B. Physical adsorption of patulin by Saccharomyces cerevisiae during fermentation. J. Food Sci. Technol. 2019, 56, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Jakopović, Ž.; Čiča, K.H.; Mrvčić, J.; Pucić, I.; Čanak, I.; Frece, J.; Pleadin, J.; Stanzer, D.; Zjalić, S.; Markov, K. Properties and fermentation activity of industrial yeasts Saccharomyces cerevisiae, S. uvarum, Candida utilis and Kluyveromyces marxianus exposed to AFB1, OTA and ZEA. Food Technol. Biotechnol. 2018, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, H.; Yang, C.; Meng, X.; Liu, B. Detoxification of mycotoxin patulin by the yeast Rhodotorula mucilaginosa. Food Control 2019, 96, 47–52. [Google Scholar] [CrossRef]
- Burgess, K.M.N.; Renaud, J.B.; McDowell, T.; Sumarah, M.W. Mechanistic insight into the biosynthesis and detoxification of fumonisin mycotoxins. ACS Chem. Biol. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Cence, K.; dos Santos, P.; Garcia, M.V.; Copetti, M.V.; Valduga, E.; Cansian, R.L.; Zeni, J.; Backes, G.T. Enzymatic biocontrol of spoilage fungi from salami. LWT 2019, 115, 108457. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal effect of engineered silver nanoparticles on phytopathogenic and toxigenic Fusarium spp. and their impact on mycotoxin accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Wang, F.; Li, Q.; He, C.; Duan, N.; Wang, Z. Assessing the toxicity in vitro of degradation products from deoxynivalenol photocatalytic degradation by using upconversion nanoparticles@ TiO2 composite. Chemosphere 2020, 238, 124648. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; de Castro Alves, L.; Alfonso, A.; Piñeirob, Y.; Vilar, S.Y.; Gomez, M.G.; Osorio, Z.V.; Sainz, M.J.; Vieytes, M.R.; Rivas, J.; et al. Detoxification agents based on magnetic nanostructured particles as a novel strategy for mycotoxin mitigation in food. Food Chem. 2019, 294, 60–66. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Dwivedy, A.K.; Singh, V.K.; Das, S.; Singh, A.; Dubey, N.K. Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci. Pollut. Res. 2019, 26, 25414–25431. [Google Scholar] [CrossRef]
- Perczak, A.; Juś, K.; Gwiazdowska, D.; Marchwińska, K.; Waśkiewicz, A. The Efficiency of Deoxynivalenol Degradation by Essential Oils under In Vitro Conditions. Foods 2019, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Aiko, V.; Mehta, A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015, 40, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montero, L.; Córdoba, J.J.; Alía, A.; Peromingo, B.; Núñez, F. Effect of Spanish smoked paprika “Pimentón de La Vera” on control of ochratoxin A and aflatoxins production on a dry-cured meat model system. Int. J. Food Microbiol. 2019, 308, 108303. [Google Scholar] [CrossRef] [PubMed]
- Kollia, E.; Proestos, C.; Zoumpoulakis, P.; Markaki, P. Capsaicin, an inhibitor of Ochratoxin A production by Aspergillus section Nigri strains in grapes (Vitis vinifera L.). Food Addit. Contam. Part. A 2019, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Mycotoxin | IARC Number * | Acronym | Fungal Species | Food Commodity | US FDA (µg/kg) | EU (µg/kg) | References |
|---|---|---|---|---|---|---|---|
| Aflatoxins B1, B2, G1, G2 | 1* | AFB1 AFB2 AFG1 AFG2 | Aspergillus flavus, Aspergillus parasiticus | Maize, wheat, rice, peanut, sorghum, pistachio, almond, ground nuts, tree nuts, figs, cottonseed, spices | 20 for total | 2–12 for B1 4–15 for total | [37] |
| Aflatoxin M1 | 2B* | AFM1 | Metabolite of aflatoxin B1 | Milk, milk products, meat | 0.5 | 0.05 in milk 0.025 in infant formulae and infant milk | [37] |
| Ochratoxin A | 2B* | OTA | Aspergillus ochraceus, Aspergillus carbonarius Penicillium verrucosum, Penicillium nordicum | Cereals, dried vine fruit, wine, grapes, coffee, cocoa, cheese | Not set | 2–10 | [37] |
| Fumonisins B1, B2, B3 | 2B* | FB1 FB2 FB3 | Fusarium verticillioides, Fusarium proliferatum | Maize, maize products, sorghum, asparagus | 2000–4000 | 200–4000 | [38] |
| Zearalenone | 3* | ZEN | Fusarium graminearum (F. roseum), Fusarium culmorum Fusarium equiseti, Fusarium cerealis, Fusarium verticillioides, Fusarium incarnatum | Cereals, cereal products, maize, wheat, barley | Not set | 20–100 | [38] |
| Trichothecenes (type B: deoxynivalenol) | 3* | DON | Fusarium graminearum, Fusarium culmorum, Fusarium cerealis | Cereals, cereal products | 1000 | 200–50 | [38,39] |
| Patulin | 3* | PAT | Penicillium expansum Bysochlamis nívea, Aspergillus clavatus, Penicillium patulum Penicillium crustosum | Apples, apple juice, and concentrate, pears, peaches, grapes, apricots, olives low acid fruit juices | 50 | 10–50 | [37,40] |
| Trichothecenes (type A: HT-2) | 3* | HT2 | Fusarium langsethiae, Fusarium sporotrichioides | Maize, wheat, barley, oat, rye | 15 | 25–1000 | [41] |
| Trichothecenes (type A: T-2 toxin) | 3* | T-2 | Fusarium langsethiae,Fusarium sporotrichioides | Maize, wheat, barley, oat, rye | 15 | 25–1000 | [41] |
| Enniatins | ENNs | Fusarium tricinctum, Fusarium avenaceum | Corn | Not set | Not set | [21] | |
| Ergot alkaloids | EAs | Claviceps purpurea, Claviceps fusiformis, Claviceps africana, Neotyphodium spp | Rye, rye-containing commodities, wheat, triticale, barley, millet and oat | Not set | Not set | [19,42] | |
| Alternariol | AOH | Alternaria alternata | Grain and grain-based products, vegetables and vegetable products, fruits and fruit products, wine and beer, oilseeds and vegetable oils | Not set | Not set | [43] |
| Mycotoxins | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoxins | 638 | 649 | 585 | 484 | 341 | 314 | 438 | 348 | 529 | 536 |
| Deoxynivalenol (DON) | 3 | 2 | 11 | 4 | 8 | 6 | 11 | |||
| Fumonisins | 1 | 3 | 4 | 4 | 7 | 3 | 5 | |||
| Ochratoxin A | 27 | 34 | 35 | 32 | 54 | 38 | 42 | 141 | 33 | |
| Patulin | 2 | |||||||||
| Zearalenone | 4 | 3 | 2 | |||||||
| Total | 669 | 688 | 635 | 525 | 410 | 364 | 500 | 489 | 529 | 569 |
| Hazard Category | Alert | Border Rejection | Information for Attention | Information for Follow-Up |
|---|---|---|---|---|
| Allergens | 158 | 11 | 35 | 3 |
| Biological contaminants (other) | 22 | 4 | 20 | |
| Food additives and flavorings | 19 | 64 | 24 | 35 |
| Foreign bodies | 106 | 10 | 19 | 33 |
| GMO food or feed | 9 | 1 | 3 | |
| Novel food | 8 | 6 | 15 | 22 |
| Metals | 56 | 13 | 55 | 9 |
| Industrial contaminants | 1 | |||
| Mycotoxins | 88 | 508 | 55 | 4 |
| Parasitic infestation | 1 | 17 | 23 | |
| Pathogenic microorganisms | 349 | 302 | 191 | 137 |
| Pesticides residues | 48 | 154 | 60 | 14 |
| Residues of veterinary medicinal products | 10 | 15 | 15 | 8 |
| Country | Food Matrix | AFs | N Samples | Incidence % | Mean μg/kg | Range μg/kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| China | Raw milk | AFM1 | 530 | 52.8 | 0.07 | 0.01–0.2 | LC-MS/MS | [82] |
| Nigeria | Breast milk | AFM1 | 40 | 77 | 0.066 | 0.001–0.601 | HPLC | [83] |
| Burkina Faso | Infant formula | AFB1 | 199 | 84 | 3.8 | 0–87.4 | HPLC | [84] |
| China | Wheat | AFB1 | 348 | 0.28 | 7.3 | <0.10–7.3 | LC-MS/MS | [85] |
| AFB2 | 348 | 0.28 | 1.2 | <0.10–1.2 | ||||
| AFG1 | 348 | 0 | − | − | ||||
| AFG2 | 348 | 0 | − | − | ||||
| China | Rice | AFB1 | 370 | 63.50 | 0.6 | 0.03–20.0 | HPLC | [86] |
| AFB2 | 370 | 17.57 | 0.2 | <0.1–3.2 | ||||
| China | Maize | AFB1 | 44 | 2.3 | 148.4 | − | HPLC | [87] |
| Turkey | Walnut | AFs | 48 | 44 | 1.33 | 0.58–15.2 | HPLC | [88] |
| Turkey | Seedless black raisins | AFs | 25 | 64 | 0.4 | 0.02–2.07 | HPLC | [89] |
| Dried figs | 45 | 51 | 1.78 | 0.16–5.20 | ||||
| Ground red peppers | 25 | 72 | 2.30 | 0.04–3.47 | ||||
| Walnuts without shell | 25 | 64 | 1.68 | 0.66–2.62 | ||||
| Nigeria | Ginger | AFs | 120 | 81 | 3.13 | 0.11–9.52 | HPLC | [90] |
| Egypt | Dried date palm | AFB1 | 28 | 4 | − | 14.4 | LC-MS/MS | [77] |
| AFB2 | 2.44 | |||||||
| Tanzania | Sun-dried sweet potato chips | AFs | 80 | 36 | 40.31 | 10.49–75.12 | HPLC | [91] |
| Ghana | Cereal-based products | AFs | 50 | 72 | − | 0.18–25.93 | HPLC | [56] |
| Czech Republic | Barley and malt samples | AFs | 52 | Nd a | − | − | LC-MS/MS | [92] |
| Country | Food Matrix | N Samples | Incidence % | Mean μg/kg | Range μg/kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|
| China | Dried vine fruits | 56 | 58.9 | 0.99 | <0.07–12.83 | HPLC | [107] |
| China | Dried fruits | 220 | 5 | 1.9 | 0.2–8.8 | LC-MS/MS | [108] |
| China | Rice | 370 | 4.87 | 0.9 | 0.30–3.2 | HPLC | [86] |
| Burkina Faso | Infant formula | 199 | 7.5 | 0.1 | 0–3.2 | HPLC | [84] |
| Syria | Fruit-based products | 12 | 33 | 0.093 | 0.019–0.156 | HPLC | [109] |
| Cereal-based baby food | 30 | 43 | 0.094 | 0.02–0.329 | |||
| Brazil | Breast milk | 86 | 0 | − | − | LC-MS/MS | [110] |
| Turkey | Eggplant | 50 | 100 | 17.67 | 8.88–21.35 | HPLC | [111] |
| Green bell pepper | 20.53 | 15.38–24.70 | |||||
| Egypt | Dried date palm | 28 | 11 | 58.7 | 1.48–6070 | LC-MS/MS | [77] |
| Lebanon | Spices | 94 | 30 | 7.1 | 2–33.9 | LC-MS/MS | [74] |
| Czech Republic | Barley and malt samples | 52 | Nd a | − | − | LC-MS/MS | [92] |
| Syria | Durum wheat | 40 | 30 | 0.6 | 0.4–0.7 | LC-MS/MS | [112] |
| China | Peanut | 28 | 7.1 | NA b | 0.25–0.65 | HPLC | [113] |
| Qatar | Baby food, cereal based | 51 | 31 | NA b | 0.05–≥0.50 | LC-MS/MS | [114] |
| Kingdom of Saudi Arabia | Cardamom | 80 | 48 | NA b | 30–78 | HPLC | [115] |
| Portugal | Roasted coffee | 6 | 33 | 1.84 μg/Kg | LOD–10.31 | HPLC | [116] |
| Ground roasted coffee | 5 | 20 | 1.45 μg/Kg | LOD–7.26 | |||
| Denmark, Kenya, Tanzania, Uganda | Roasted coffee | 57 | 46 | NA b | 2.3 | UHPLC-MS/MS | [117] |
| Country | Food Matrix | Fumonisins | N Samples | Incidence % | Mean μg/kg | Range μg/kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| China | Maize | FB1 | 44 | 100 | 116.5 | 16.5–315.9 | HPLC | [89] |
| Burkina Faso | Infant formula | FB1 + FB2 | 199 | 1.5 | 30.3 | 0–672.9 | HPLC | [86] |
| United States | Infant cereal-based products | FB1 | 64 | 2 | NA | <LOD–6.2 | LC-MS/MS | [136] |
| FB2 | 8 | <LOD–15.8 | ||||||
| Egypt | Dates palm | FB2 | 28 | 7 | 10.6 | 4.99–16.2 | LC-MS/MS | [77] |
| Serbia | Crop maize | FUs | 90 | 100 | 1730 | 520.0–5800.0 | ELISA | [137] |
| China | Corn flakes | FB1 | 14 | 100 | 104.1 | 1.0–171.0 | UPLC-MS-MS | [138] |
| FB2 | 14 | 93 | 14.2 | <0.27–25.6 | ||||
| FB3 | 14 | 93 | 17.3 | <0.27–31.5 | ||||
| Lebanon | Spices | FB1 | 94 | 64 | 6432.3 | 18.2–113474.5 | LC-MS/MS | [74] |
| FB2 | 35 | 230.2 | 15.1–1757.4 | |||||
| Argentina | Wheat-based products | FB1 + FB2 | 91 | 74 | 1.54 | 0.05–18.9 | HPLC-MS/MS | [132] |
| China | Wheat flour | FB1 | 369 | 6.2 | NA | 0.3–34.6 | UPLC-MS/MS | [138] |
| Tanzania | Sun-dried sweet potato chips | FB1 | 80 | 97.5 | 44.69 | 29.34–628.78 | HPLC | [91] |
| Brazil | Wheat cultivars | FB1 | 11 | 54 | 2814.33 | 958–4906 | HPLC | [139] |
| Argentina | Wheat | FB1 | 135 | 97 | 30.07 | 0.16–680.4 | HPLC-MS/MS | [133] |
| Durum wheat | 40 | 77.5 | 84.75 | 0.15–1304.4 | ||||
| Wheat | FB2 | 135 | 51 | 2.88 | 0.25–23.7 | |||
| Durum wheat | 40 | 42.5 | 10.47 | 0.43–47 | ||||
| Italy | Durum wheat | FB1 | 4 | 100 | 33 | 15–51 | HPLC-MS/MS | [140] |
| Syria | Durum wheat | FB1 | 40 | 10 | 5 | 5–6 | HPLC-MS/MS | [112] |
| Country | Food Matrix | TCs | N Samples | Incidence % | Mean μg/kg | Range μg/kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| China | Wheat | DON | 38 | 100 | 106.5 | 0.5–604.0 | LC-MS/MS | [157] |
| China | Maize | DON | 44 | 65.9 | 831.0 | 5.8–9843.3 | HPLC | [87] |
| Romania | Wheat | DON | 31 | 26 | 748 | 110–1787 | LC-MS/MS | [158] |
| Flour | 35 | 3 | 190 | 190 | ||||
| Pakistan | Rice | DON | 180 | 8 | 6.99 | <LOD | LC-MS/MS | [159] |
| Brazil | Wheat | DON | 48 | 100 | 2398 | 1329–3937 | HPLC | [160] |
| Italy | Wheat | DON | 293 | 97 | NA a | 56–27088 | GC-MS | [161] |
| Brazil | Wheat | DON | 53 | 47 | 641 | 243–2281 | HPLC | [162] |
| China | Wheat | DON | 348 | 91 | 240 | 240–1129 | LC-MS/MS | [85] |
| Iran | Wheat | DON | 96 | 83 | 630 | 23–1270 | [163] | |
| Finland | Wheat | DON | 30 | NA a | 866 | NA–5510 | LC-MS/MS | [164] |
| Brazil | Wheat | DON | 58 | 91 | 360 | NA–1310 | HPLC | [165] |
| Brazil | Wheat | DON | 745 | 86 | 1690 | NA–8501 | HPLC | [166] |
| 407 | NA–2419 | |||||||
| Brazil | Wheat | DON | 150 | 99 | 706 | 183–2150 | HPLC | [167] |
| Hungary | Wheat | DON | 29 | 72 | NA a | NA–1880 | ELISA | [168] |
| Argentina | Wheat | DON | 84 | 100 | 1750 | NA–9480 | LC-MS/MS | [169] |
| Switzerland | Wheat | DON | 686 | 80 | 607 | NA–10600 | LC-MS/MS | [170] |
| Brazil | Wheat | DON | 172 | 77 | 234 | 73–2794 | HPLC | [171] |
| Poland | Wheat | DON | 92 | 83 | 140 | 10–1265 | HPLC | [172] |
| China | Wheat | DON | 181 | 82 | 500 | 33–3030 | HPLC | [173] |
| Hungary | Maize | DON | 29 | 86 | 1872 | 225–2963 | ELISA | [168] |
| T-2 | 29 | 55 | 69 | NA–146 | ||||
| Serbia | Corn flour | DON | 56 | 42.9 | 101 | NA–931 | HPLC | [174] |
| Cornflakes | 15 | 40 | 255 | NA–878 | ||||
| Brazil | Barley | DON | 76 | 94 | 310–15,500 | 1700–7500 | LC-MS/MS | [175] |
| Brazil | Bakery Products | DON | 36 | 100 | 591 | 60–1720 | HPLC | [165] |
| India | Infant Foods | DON | 29 | 66 | NA a | 5–228 | ELISA | [176] |
| Germany | Noodles and Pasta | DON | 40 | 97 | 387 | 60–1609 | HPLC | [165] |
| Finland | Oat | DON | 31 | 100 | NA a | 23,800 | LC-MS/MS | [164] |
| UK | Oat | DON | 303 | 32 | 28 | NA–1866 | LC-MS/MS | [177] |
| Finland | Oat | DON | 1672 | 79 | NA a | NA–21608 | GC-MS | [178] |
| Poland | Oat | DON | 147 | 31 | NA a | NA–2975 | HPLC-HRMS | [179] |
| Tunisia | Cereal-based products | HT-2 | 32 | 3 | 1 | <LOD–209 | LC-MS/MS | [153] |
| Lebanon | Wheat grains, wheat flour, and bread | HT-2 | 137 | 0 | − | − | LC-MS/MS | [180] |
| T-2 | ||||||||
| Spain | Cereal-based food, beer | HT-2 | 479 | NA a | 0.047–0.214 | NA a | GC-ECD | [181] |
| T-2 | 479 | 0.006 | 0.215–0.072 | NA a | ||||
| Spain | Wheat semolina | HT-2 | 15 | 33.3 | 8.9 | 6.7–15.2 | GC–QqQ-MS/MS | [182] |
| Country | Food Matrix | N Samples | Incidence% | a Mean μg/Kg | a Range μg/Kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|
| China | Wheat | 348 | 13.2 | 8.4 | <0.25-98.8 | LC-MS/MS | [85] |
| China | Maize | 50 | 94 | 109.1 | 0.2-3613.0 | LC-MS/MS | [192] |
| China | Maize | 44 | 13.6 | 50.8 | 40.7-1056.8 | HPLC | [87] |
| Hungary | Maize | 29 | 41 | 267 | NAa-565 | ELISA | [168] |
| South Korea | Infant formula | 36 | 25 | NAa | 3.3-17.6 | UHPLC | [193] |
| Serbia | Crop maize | 90 | 0 | - | - | ELISA | [137] |
| Pakistan | Rice | 180 | 15 | 8.48 | NAa | LC–MS/MS | [159] |
| Lebanon | Spices | 94 | 30 | 30.6 | 0.4-305.4 | LC-MS/MS | [74] |
| Brazil | Wheat flour | 39 | 2.6 | - | 26.7 | UPLC–MS/MS | [194] |
| China | Peanut | 28 | 36 | NAa | 0.09–26.8 | HPLC | [113] |
| China | Grapes | 36 | 6 | NAa | 0.29-0.36 | UHPLC–MS/MS | [75] |
| Wine | 42 | 7 | NAa | <LOQ to 1.85 | |||
| Syria | Wheat | 40 | 25 | 13 | 4-34 | LC–MS/MS | [112] |
| Italy | Wheat | 47 | 34 | 44 | 7-230 | HPLC–MS/MS | [112] |
| Qatar | Baby food, milk based | 51 | 37 | NA | 1 - ≥5 | HPLC | [114] |
| Country | Food Matrix | Emerging Fusarium mycotoxins | N Samples | a Incidence % | Mean μg/Kg | b Range μg/Kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| Serbia | Maize | FP | 21 | 76 | - | 85.4–1121 | LC-MS/MS | [196] |
| Japan | Corn | BEA | 44 | 34 | 3.8 | NAb-26.1 | LC-MS/MS | [202] |
| Wheat flour | 66 | Nda | - | - | ||||
| Wheat flour | ENB | 66 | 81.8 | 33.4 | NAb-237 | |||
| Italy | Wheat | BEA | 43 | 14 | 0.0004 | 0.0018-0.0051 | HPLC–MS/MS | [112] |
| Syria | Wheat | BEA | 40 | 13 | 0.0002 | 0.0015-0.0017 | HPLC–MS/MS | [112] |
| Italy | Wheat | BEA | 54 | 67 | - | 0.04-5.28 | LC-MS/MS | [211] |
| Italy | Durum wheat | BEA | 108 | 94 | - | 0.03-56.40 | LC-MS/MS | [211] |
| Morocco | Wheat | BEA | 80 | 10 | 0.9 | NAb-16 | LC-MS/MS | [205] |
| ENA | 14 | 6.8 | NAb-75 | |||||
| ENA1 | 18 | 13 | NAb-134 | |||||
| ENB | 61 | 57 | NAb-2570 | |||||
| ENB1 | 53 | 27 | NAb-345 | |||||
| Serbia | Maize | ENNs | 29 | 10 | - | 0.12–0.47 | LC-MS/MS | [212] |
| Italy | Wheat | ENA | 43 | 14 | 0.0012 | 0.0031-0.0181 | HPLC–MS/MS | [112] |
| Syria | Wheat | ENA | 40 | 10 | 0.0002 | 0.0015-0.0022 | HPLC–MS/MS | [112] |
| Country | Food Matrix | N Samples | a,b Incidence% | b Mean μg/Kg | Range μg/Kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|
| Albania | Wheat | 35 | 48.6 | 337.2 | 17.3–975.4 | LC-MS/ MS | [215] |
| Canada | Barley | 67 | 73 | 1150.50 | 2.21-29424.6 | LC-MS/ MS | [214] |
| Italy | Rye-based products | 16 | 85 | NAb | 2.6–188.6 | LC-MS/MS | [42] |
| Wheat-based products | 55 | 2.5– 1142.6 | |||||
| China | Cereal samples | 123 | Nd a | - | - | LC-MS/MS | [219] |
| Canada | Cereals | 228 | NA b | NAb | 65-1140 | LC-MS/MS | [216] |
| Country | Food Matrix | Alternaria Toxins | N Samples | aIncidence % | bMean μg/Kg | Range μg/Kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| China | Dried fruits | AOH | 220 | 2.3 | 12.0 | 3.5–27.4 | LC-MS/ MS | [108] |
| TeA | 42.7 | 465.5 | 6.9–5665.3 | |||||
| AME | 8.2 | 3.0 | 0.2–15.0 | |||||
| TEN | 20.5 | 120.5 | 1.4–1032.6 | |||||
| China | Wheat | TeA | 370 | 100 | 289.0 | 6.0–3330.7 | LC-MS/ MS | [226] |
| AOH | 370 | 47 | 12.9 | 1.3–74.4 | ||||
| AME | 370 | 15 | 9.1 | 0.3–54.8 | ||||
| Argentina | Flour | TeA | 23 | 61 | 7360 | LOQ-17,719 | HPLC-DAD | [224] |
| Wheat | 21 | 57.1 | 19,190 | LOQ-92,000 | ||||
| Bran | 21 | 66.7 | 16,760 | LOQ-82,600 | ||||
| Italy | Dried tomatoes | TeA | 10 | 100 | 16,291 | 425–81,592 | LC-MS/MS | [227] |
| Fresh tomatoes | 8 | 100 | 1,010 | 11–4560 | ||||
| Germany | Juice samples | TeA | 88 | 62 | NAb | 1.4–19.2 | LC-MS/MS | [223] |
| Red wine | AME | 11 | Nda | - | - | |||
| China | Wheat flour | TeA | 181 | 99.4 | 88.4 | 1.76–520 | UPLC-MS/MS | [228] |
| Dried noodle | 52 | 96.2 | 47.6 | 4.86–158 | ||||
| Bread | 50 | 98 | 11.7 | 1.95–38.2 | ||||
| Steamed bread | 40 | 100 | 21.2 | 6.56–46.3 |
| Country | Food Matrix | N Samples | aIncidence % | aMean μg/kg | Range μg/kg | Detection Technique | Reference |
|---|---|---|---|---|---|---|---|
| China | Dried fig | 20 | 65.0 | - | 15.0-276.9 | HPLC | [240] |
| Raisins and other dried fruits | 36 | 8.3 | - | 12.5-68.0 | |||
| Juice | 20 | 10.0 | - | 8.0-16.8 | |||
| Jam | 20 | 30.7 | - | 8.6-11.0 | |||
| China | Dried fruits source | 220 | 0.5 | 30.6 | 30.6 | LC-MS/ MS | [108] |
| China | Apple juice | 23 | 39.1 | 1.0 | 0.50-4.82 | LC-MS/ MS | [241] |
| Mix fruit juice | 20 | 20 | 0.74 | 0.50-2.46 | |||
| Qatar | Fruit-based products | 13 | 100 | 20.8 | 1.02-61.3 | LC-MS/MS | [242] |
| Apple juice | 20 | 100 | NAa | 5.27-82.21 | |||
| Tunisia | Fruit-based products | 214 | 50 | 89 | 2-889 | HPLC | [243] |
| Pakistan | Apple | 36 | 75 | NAa | <LOD–630.8 | HPLC | [239] |
| China | Apple juice (including organic and conventional apple juice and juice concentrate) | 4 | NAa | NAa | <LOD–16.8 | HPLC | [240] |
| Serbia | Apple juice (including organic and conventional apple juice and juice concentrate) | 73 | 74 | NAa | <LOD–65.4 | HPLC | [244] |
| Argentina | Apple juice (including organic and conventional apple juice and juice concentrate) | 4634 | 40 | 26 | <LOD–19,622 | HPLC | [245] |
| Malaysia | Apple juice (including organic and conventional apple juice and juice concentrate) | 13 | 8 | NAa | <LOD–26.9 | HPLC | [246] |
| Italy | Products for babies (including apple juice, apple sauce, and compotes) | 26 | 0 | - | - | HPLC | [247] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. https://doi.org/10.3390/foods9020137
Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods. 2020; 9(2):137. https://doi.org/10.3390/foods9020137
Chicago/Turabian StyleAgriopoulou, Sofia, Eygenia Stamatelopoulou, and Theodoros Varzakas. 2020. "Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods" Foods 9, no. 2: 137. https://doi.org/10.3390/foods9020137
APA StyleAgriopoulou, S., Stamatelopoulou, E., & Varzakas, T. (2020). Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods, 9(2), 137. https://doi.org/10.3390/foods9020137







