Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility
Abstract
:1. Introduction
2. Cell Wall: The Main Contributor to the Release of Food Constituents
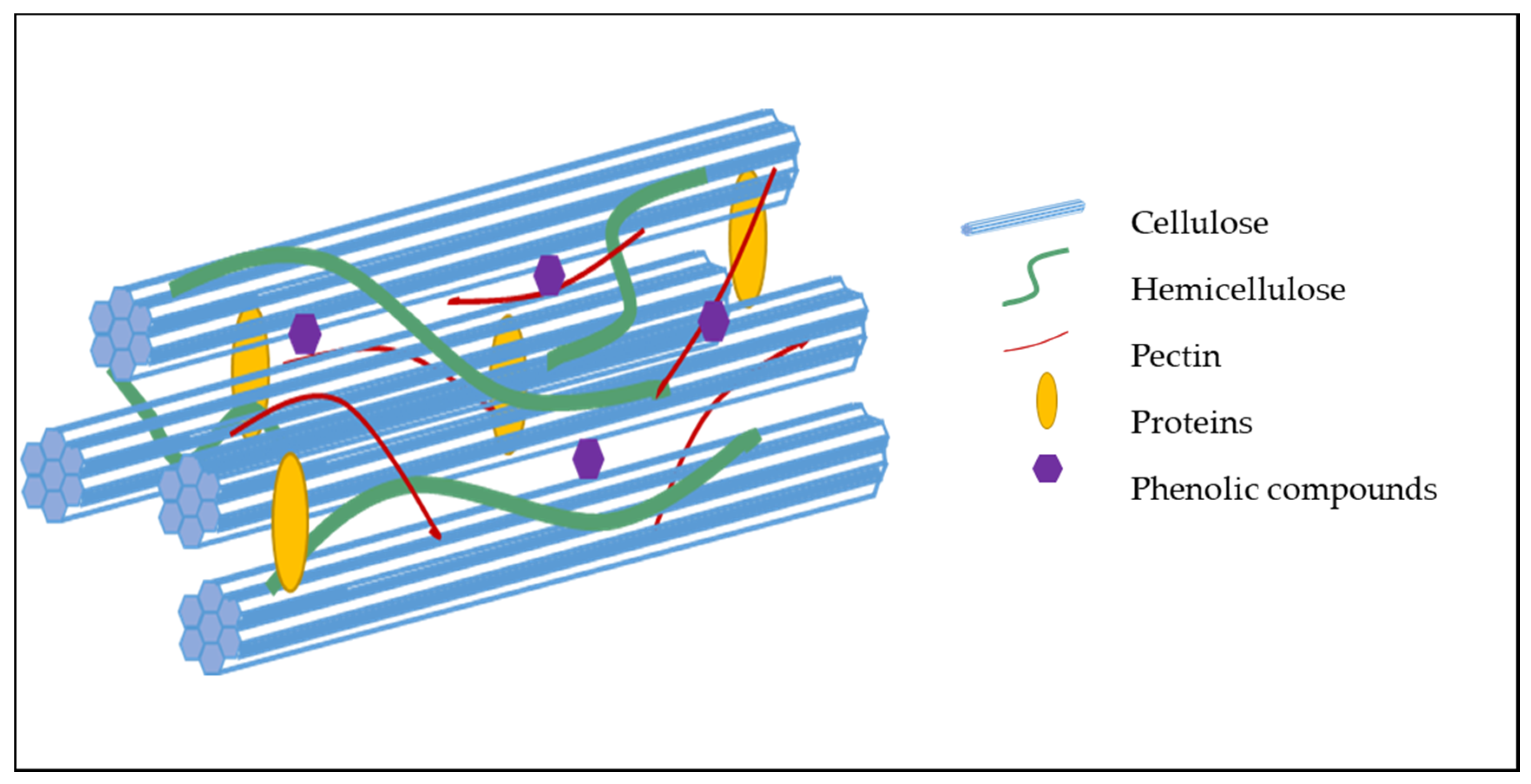
2.1. Molecular Composition of the Cell Wall
2.2. Organization and Interaction Between Cell Wall Components
2.3. Porosity
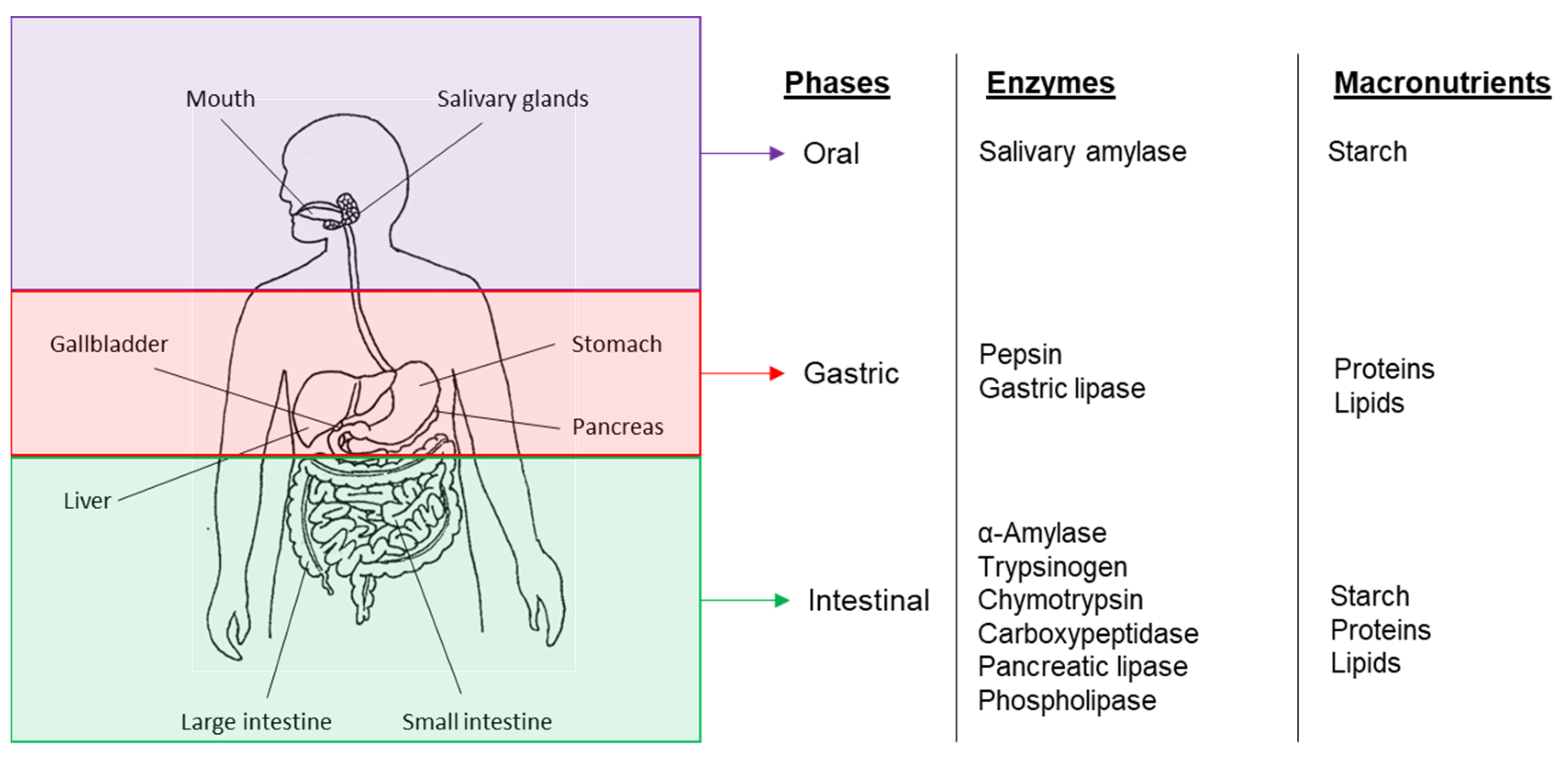
3. Bioaccessibility and Digestion of Plant-Based Food Components
3.1. Cell Wall Properties and Food Structure
3.2. Encapsulation
3.3. Solubility and Resulting Properties (Viscosity)
3.4. Sequestration
4. Characterization of Plant-Based Foods and Cell Wall Materials
4.1. Overall Appraisal of the Food Structure
4.2. Characteristics at the Cellular and Molecular Levels
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wills, R.B.H.; McGlasson, W.B.; Graham, D.; Joyce, D.C. Structure and composition. Postharvest: An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals, 5th ed.; CABI: Wallingford, UK, 2007; pp. 15–27. [Google Scholar]
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, Diet, and Health. Annu. Rev. Plant Biol. 2013, 64, 19–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.R.; Early, M.P. Principles of Horticulture, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palafox-Carlos, H.; Ayala-Zavala, J.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Casado, A. The health potential of fruits and vegetables phytochemicals: Notable examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef]
- Brummer, E.C.; Barber, W.T.; Collier, S.; Cox, T.S.; Johnson, R.; Murray, S.C.; Olsen, R.T.; Pratt, R.C.; Thro, A.M. Plant breeding for harmony between agriculture and the environment. Front. Ecol. Environ. 2011, 9, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Brett, C.; Waldron, K. Physiology and Biochemistry of Plant Cell Walls, 2nd ed.; Black MaC, B., Ed.; Chapman & Hall: Cambridge, MA, USA, 1996. [Google Scholar]
- Waldron, K.; Parker, M.L.; Smith, A. Plant Cell Walls and Food Quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 101–119. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- McDougall, G.J.; Morrison, I.M.; Stewart, D.; Hillman, J.R. Plant cell walls as dietary fibre: Range, structure, processing and function. J. Sci. Food Agri. 1996, 70, 133–150. [Google Scholar] [CrossRef]
- Wang, T.; Hong, M. Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 2016, 67, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorshkova, T.; Mikshina, P.; Gurjanov, O.P.; Chemikosova, S.B. Formation of plant cell wall supramolecular structure. Biochemistry (Moscow) 2010, 75, 159–172. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Hydrophobic Interaction in the Gelation of High Methoxyl Pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Frankova, L.; Fry, S.C. Phylogenetic variation in glycosidases and glycanases acting on plant cell wall polysaccharides, and the detection of transglycosidase and trans-β-xylanase activities. Plant J. 2011, 67, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.; Michel, G.; Herve, C.; Domozych, D.S.; Willats, W.; Tuohy, M.G.; Kloareg, B.; Stengel, D. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary-cell walls in flowering plants—Consistency of molecular-structure with the physical-properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- O’Neill, M.A.; York, W.S. The composition and structure of plant primary cell walls. In The Plant Cell Wall; Rose, J.K.C., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–54. [Google Scholar]
- Pauly, M.; Albersheim, P.; Darvill, A.; York, W.S. Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J. 1999, 20, 629–639. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Fry, S.C.; Mohler, K.E.; Nesselrode, B.H.; Frankova, L. Mixed-linkage β-glucan: Xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J. 2008, 55, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef] [PubMed]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Baron-Epel, O.; Gharyal, P.K.; Schindler, M. Pectins as mediators of wall porosity in soybean cells. Planta 1988, 175, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the Pore Size of Cell Walls of Living Plant Cells. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Ehwald, R.; Woehlecke, H.; Titel, C. Cell wall microcapsules with different porosity from suspension cultured Chenopodium album. Phytochem. 1992, 31, 3033–3038. [Google Scholar] [CrossRef]
- Bhattarai, R.R.; Dhital, S.; Mense, A.; Gidley, M.J.; Shi, Y.-C. Intact cellular structure in cereal endosperm limits starch digestion in vitro. Food Hydrocoll. 2018, 81, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, R.R.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. Digestion of isolated legume cells in a stomach-duodenum model: Three mechanisms limit starch and protein hydrolysis. Food Funct. 2017, 8, 2573–2582. [Google Scholar] [CrossRef]
- Dhital, S.; Bhattarai, R.R.; Gorham, J.; Gidley, M.J. Intactness of cell wall structure controls the in vitro digestion of starch in legumes. Food Funct. 2016, 7, 1367–1379. [Google Scholar] [CrossRef]
- Do, D.T.; Singh, J.; Oey, I.; Singh, H. Modulating effect of cotyledon cell microstructure on in vitro digestion of starch in legumes. Food Hydrocoll. 2019, 96, 112–122. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Carrière, F.; Mackie, A.; Gray, D.; Butterworth, P.J.; Ellis, P.R. The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds. Food Funct. 2016, 7, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Gidley, M.J.; Dhital, S. Wall porosity in isolated cells from food plants: Implications for nutritional functionality. Food Chem. 2019, 279, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Pallares, A.P.; Miranda, B.A.; Truong, N.Q.A.; Kyomugasho, C.; Chigwedere, C.M.; Hendrickx, M.E.G.; Grauwet, T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018, 9, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. A closer look to cell structural barriers affecting starch digestibility in beans. Carbohydr. Polym. 2018, 181, 994–1002. [Google Scholar] [CrossRef]
- Mandalari, G.; Parker, M.L.; Grundy, M.M.-L.; Grassby, T.; Smeriglio, A.; Bisignano, G.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection. Nutrients 2018, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Rongkaumpan, G.; Amsbury, S.; Andablo-Reyes, E.; Linford, H.; Connell, S.; Knox, J.P.; Sarkar, A.; Benitez-Alfonso, Y.; Orfila, C. Cell Wall Polymer Composition and Spatial Distribution in Ripe Banana and Mango Fruit: Implications for Cell Adhesion and Texture Perception. Front. Plant Sci. 2019, 10, 858. [Google Scholar] [CrossRef]
- Zahir, M.A.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [Green Version]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [Green Version]
- Davenport, H.W. Physiology of the Digestive Tract: An Introductory Text; Year Book Medical Publishers: Chicago, IL, USA, 1982. [Google Scholar]
- Joardder, M.U.; Kumar, C.; Karim, M.A. Food structure: Its formation and relationships with other properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1190–1205. [Google Scholar] [CrossRef]
- Blackwood, A.; Salter, J.; Dettmar, P.; Chaplin, M. Dietary fibre, physicochemical properties and their relationship to health. J. R. Soc. Promot. Heal. 2000, 120, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ellis, P.R. Oat beta-glucan: Physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br. J. Nutr. 2014, 112 (Suppl 2), S4–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikeman, C.L.; Fahey, G.C. Viscosity as Related to Dietary Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Gidley, M.J.; Yakubov, G. Functional categorisation of dietary fibre in foods: Beyond ‘soluble’ vs. ‘insoluble’. Trends Food Sci. Technol. 2019, 86, 563–568. [Google Scholar] [CrossRef]
- Knudsen, K.B. The nutritional significance of “dietary fibre” analysis. Anim. Feed. Sci. Technol. 2001, 90, 3–20. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Elsevier Science & Technology: Amsterdam, The Netherlands, 2016; 1226p. [Google Scholar]
- Swackhamer, C.; Bornhorst, G.M. Fracture properties of foods: Experimental considerations and applications to mastication. J. Food Eng. 2019, 263, 213–226. [Google Scholar] [CrossRef]
- Rabiti, D.; Orfila, C.; Holmes, M.; Bordoni, A.; Sarkar, A. In vitro oral processing of raw tomato: Novel insights into the role of endogenous fruit enzymes. J. Texture Stud. 2018, 49, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Jolie, R.P.; Christiaens, S.; De Roeck, A.; Fraeye, I.; Houben, K.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E.G. Pectin conversions under high pressure: Implications for the structure-related quality characteristics of plant-based foods. Trends Food Sci. Technol. 2012, 24, 103–118. [Google Scholar] [CrossRef]
- Binner, S.; Jardine, W.G.; Renard, C.M.C.G.; Jarvis, M.C. Cell wall modifications during cooking of potatoes and sweet potatoes. J. Sci. Food Agric. 2000, 80, 216–218. [Google Scholar] [CrossRef]
- Ng, A.; Waldron, K.W. Effect of Cooking and Pre-Cooking on Cell-Wall Chemistry in Relation to Firmness of Carrot Tissues. J. Sci. Food Agric. 1997, 73, 503–512. [Google Scholar] [CrossRef]
- Codex Alimentarius. Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission; FAO: Rome, Italy, 2010. [Google Scholar]
- Brummer, Y.; Kaviani, M.; Tosh, S. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Quint, J.; Rieder, A.; Balance, S.; Dreiss, C.A.; Butterworth, P.J.; Ellis, P.R. Impact of hydrothermal and mechanical processing on dissolution kinetics and rheology of oat β-glucan. Carbohydr. Polym. 2017, 166, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, M.M.-L.; Quint, J.; Rieder, A.; Balance, S.; Dreiss, C.A.; Cross, K.L.; Gray, R.; Bajka, B.; Butterworth, P.J.; Ellis, P.R.; et al. The impact of oat structure and β-glucan on in vitro lipid digestion. J. Funct. Foods 2017, 38 Pt A, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Poutanen, K.S.; Fiszman, S.; Marsaux, C.F.M.; Pentikäinen, S.P.; Steinert, R.; Mela, D.J. Recommendations for characterization and reporting of dietary fibers in nutrition research. Am. J. Clin. Nutr. 2018, 108, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.H.; Grundy, M.M.-L.; Grassby, T.; Vasilopoulou, D.; Frost, G.S.; Butterworth, P.J.; Berry, S.E.; Sanderson, J.; Ellis, P.R. Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: A randomized controlled trial in healthy ileostomy participants. Am. J. Clin. Nutr. 2015, 102, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.H.; Maillot, M.; Parker, R.; Warren, F.J. A comparison of the kinetics of in vitro starch digestion in smooth and wrinkled peas by porcine pancreatic alpha-amylase. Food Chem. 2018, 244, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Grundy, M.M.-L.; Wilde, P.J.; Butterworth, P.J.; Gray, R.; Ellis, P.R. Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis. Food Chem. 2015, 185, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Merali, Z.; Ryden, P.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Lagana, G.; Faulks, R.M.; Waldron, K.W. Durum wheat particle size affects starch and protein digestion in vitro. Eur. J. Clin. Nutr. 2018, 57, 319–325. [Google Scholar] [CrossRef]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Influence of particle size and intestinal conditions on in vitro lipid and protein digestibility of walnuts and peanuts. Food Res. Int. 2019, 119, 951–959. [Google Scholar] [CrossRef]
- Verkempinck, S.; Pallares Pallares, A.; Hendrickx, M.; Grauwet, T. Processing as a tool to manage digestive barriers in plant-based foods: Recent advances. Curr. Opin. Food Sci. 2020, 35, 1–9. [Google Scholar] [CrossRef]
- Tydeman, E.A.; Parker, M.L.; Wickham, M.S.J.; Rich, G.T.; Faulks, R.M.; Gidley, M.J.; Fillery-Travis, A.; Waldron, K.W. Effect of Carrot (Daucus carota) Microstructure on Carotene Bioaccessibilty in the Upper Gastrointestinal Tract. In Vitro Simulations of Carrot Digestion. J. Agric. Food Chem. 2010, 58, 9847–9854. [Google Scholar] [CrossRef] [PubMed]
- Chigwedere, C.M.; Olaoye, T.F.; Kyomugasho, C.; Kermani, Z.J.; Pallares, A.P.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E.G. Mechanistic insight into softening of Canadian wonder common beans (Phaseolus vulgaris) during cooking. Food Res. Int. 2018, 106, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chem. 2019, 286, 557–566. [Google Scholar] [CrossRef]
- Capuano, E.; Pellegrini, N.; Ntone, E.; Nikiforidis, C.V. In vitro lipid digestion in raw and roasted hazelnut particles and oil bodies. Food Funct. 2018, 9, 2508–2516. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Walker, A.W.; Vermeiren, J.; Sheridan, P.O.; Bosscher, D.; Garcia-Campayo, V.; Parkhill, J.; Flint, H.J.; Dumcan, S.H. Impact of carbohydrate substrate complexity on the diversity of the human colonic microbiota. FEMS Microbiol. Ecol. 2019, 95, fiy201. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, D.; Fogliano, V. Food Design to Feed the Human Gut Microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef] [Green Version]
- Ellis, P.R.; Kendall, C.W.C.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J.A. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Besten, G.D.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: Structure and solubility. In Solubility of Polysaccharides; Xu, Z., Ed.; InTech: Rijeka, Croatia, 2017; pp. 7–21. [Google Scholar]
- Morris, E.R. Assembly and rheology of non-starch polysaccharides. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2001; pp. 30–41. [Google Scholar]
- Wood, P.J. Relationships between solution properties of cereal β-glucans and physiological effects—A review. Trends Food Sci. Technol. 2002, 15, 313–320. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Fardet, A.; Tosh, S.M.; Rich, G.T.; Wilde, P.J. Processing of oat: The impact on oat’s cholesterol lowering effect. Food Funct. 2018, 9, 1328–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C.S.; Blake, D.E.; Ellis, P.R.; Schofield, J.D. Effects of guar galactomannan on wheat bread microstructure and on the in vitro and in vivo digestibility of starch in bread. J Cell Sci. 1996, 24, 151–160. [Google Scholar]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of alpha-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Flanagan, B.M.; Mata, J.P.; Gilbert, E.P.; Gidley, M.J. Molecular interactions of a model bile salt and porcine bile with (1,3:1,4)-beta-glucans and arabinoxylans probed by 13C NMR and SAXS. Food Chem. 2016, 197 Pt A, 676–685. [Google Scholar] [CrossRef] [Green Version]
- Pabois, O.; Antoine-Michard, A.; Zhao, X.; Omar, J.; Ahmed, F.; Alexis, F.; Harvey, R.D.; Grillo, I.; Gerelli, Y.; Grundy, M.M.-L.; et al. Interactions of bile salts with a dietary fibre, methylcellulose, and impact on lipolysis. Carbohyd. Polym. 2020, 231, 115741. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Naumann, S.; Schweiggert-Weisz, U.; Bader-Mittermaier, S.; Haller, D.; Eisner, P. Differentiation of Adsorptive and Viscous Effects of Dietary Fibres on Bile Acid Release by Means of In Vitro Digestion and Dialysis. Int. J. Mol. Sci. 2018, 19, 2193. [Google Scholar] [CrossRef] [Green Version]
- Lentle, R.G.; Janssen, P.W.M. Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: A review. J. Comp. Physiol. B 2008, 178, 673–690. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Plankeele, J.-M.; Domoney, C.; Warren, F.J. Ultra-high performance liquid chromatography-size exclusion chromatography (UPLC-SEC) as an efficient tool for the rapid and highly informative characterisation of biopolymers. Carbohydr. Polym. 2018, 196, 422–426. [Google Scholar] [CrossRef]
- Rieder, A.; Knutsen, S.H.; Ulset, A.S.; Christensen, B.E.; Andersson, R.; Mikkelson, A.; Tuomainen, P.; Maina, N.; Balance, S. Inter-laboratory evaluation of SEC-post-column calcofluor for determination of the weight-average molar mass of cereal beta-glucan. Carbohyd. Polym. 2015, 124, 254–264. [Google Scholar] [CrossRef]
- Dürrenberger, M.B.; Handschin, S.; Conde-Petit, B.; Escher, F. Visualization of Food Structure by Confocal Laser Scanning Microscopy (CLSM). LWT 2001, 34, 11–17. [Google Scholar] [CrossRef]
- Kaláb, M.; Allan-Wojtas, P.; Miller, S. Microscopy and other imaging techniques in food structure analysis. Trends Food Sci. Technol. 1995, 6, 177–186. [Google Scholar] [CrossRef]
- Comino, P.; Collins, H.M.; Lahnstein, J.; Gidley, M.J. Effects of diverse food processing conditions on the structure and solubility of wheat, barley and rye endosperm dietary fibre. J. Food Eng. 2016, 169, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Korompokis, K.; De Brier, N.; Delcour, J.A. Differences in endosperm cell wall integrity in wheat (Triticum aestivum L.) milling fractions impact on the way starch responds to gelatinization and pasting treatments and its subsequent enzymatic in vitro digestibility. Food Funct. 2019, 10, 4674–4684. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.; Ellis, P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.S.; Fulcher, R.G. Microstructure and chemistry of the oat kernel. In Oats: Chemistry and Technology; Webster, F.H., Wood, P.J., Eds.; American Association of Cereal Chemists, Inc. (AACC): St. Paul, MN, USA, 2011; pp. 77–94. [Google Scholar]
- Pathan, A.; Bond, J.; Gaskin, R. Sample preparation for scanning electron microscopy of plant surfaces—Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef]
- Yu, P.; Block, H.; Niu, Z.; Doiron, K. Rapid characterization of molecular chemistry, nutrient make-up and microlocation of internal seed tissue. J. Synchrotron Radiat. 2007, 14, 382–390. [Google Scholar] [CrossRef]
- Sene, C.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy (An Investigation of Five Higher Plant Cell Walls and Their Components). Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Warren, F.J.; Perston, B.B.; Galindez-Najera, S.P.; Edwards, C.H.; Powell, P.O.; Mandalari, G.; Campbell, G.M.; Butterworth, P.J.; Ellis, P.R. Infrared microspectroscopic imaging of plant tissues: Spectral visualization of Triticum aestivum kernel and Arabidopsis leaf microstructure. Plant J. 2015, 84, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing cellulose structures with vibrational spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ha, N.; Ou, J.Z.; Berean, K.J. Ingestible Sensors. ACS Sensors 2017, 2, 468–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackie, A.; Bajka, B.; Rigby, N.M.; Wilde, P.J.; Alves-Pereira, F.; Mosleth, E.F.; Rieder, A.; Kirkhus, B.; Salt, L.J. Oatmeal particle size alters glycemic index but not as a function of gastric emptying rate. Am. J. Physiol. Liver Physiol. 2017, 313, G239–G246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, L.M.; Kehoe, J.J.; Barry, L.; Buckley, M.J.M.; Shanahan, F.; Mok, K.H.; Brodkorb, A. Gastric digestion of α-lactalbumin in adult human subjects using capsule endoscopy and nasogastric tube sampling. Br. J. Nutr. 2014, 112, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciani, L.; Gowland, P.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Fillery-Travis, A. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Liver Physiol. 2001, 280, G1227–G1233. [Google Scholar] [CrossRef]
- McCleary, B.V.; Ames, N.; Cox, J.; Iilians, S.; Jin, Y.; Johnson, M.; McCarthy, S.; McKie, V.; Nishibata, T.; Pastell, H.; et al. Total Dietary Fiber (CODEX Definition) in Foods and Food Ingredients by a Rapid Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study, First Action 2017.16. J. AOAC Int. 2019, 102, 196–207. [Google Scholar] [CrossRef] [Green Version]
- McCleary, B.V.; McLoughlin, C.; Charmier, L.M.J.; McGeough, P. Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products. Cereal Chem. 2020, 97, 114–137. [Google Scholar] [CrossRef] [Green Version]
- Moller, I.; Sørensen, I.; Bernal, A.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef]
- Kirby, A.; Gunning, A.P.; Morris, V.J. Imaging polysaccharides by atomic force microscopy. Biopolymers 1996, 38, 355–366. [Google Scholar] [CrossRef]
- Kirby, A.; Gunning, A.; Waldron, K.; Morris, V.; Ng, A. Visualization of plant cell walls by atomic force microscopy. Biophys. J. 1996, 70, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Pettolino, F.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Price, N.P. Permethylation linkage analysis techniques for residual carbohydrates. Appl. Biochem. Biotechnol. 2008, 148, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Cellulose-Pectin Spatial Contacts Are Inherent to Never-Dried Arabidopsis Primary Cell Walls: Evidence from Solid-State Nuclear Magnetic Resonance. Plant Physiol. 2015, 168, 871–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyland, L.L.; Taraban, M.B.; Yu, Y.B. Using small-angle scattering techniques to understand mechanical properties of biopolymer-based biomaterials. Soft Matter 2013, 9, 10218–10228. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.S. Small-Angle Scattering Techniques (SAXS/SANS). In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 95–111. [Google Scholar]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [Green Version]


| physicochemical Property | Definition | Main Factors Affecting the Property | Dietary Fiber |
|---|---|---|---|
| Solubility | Ability of a polysaccharide in a solid form or contained in a solid food matrix to disperse in a liquid (often water) and form a homogeneous dispersion.Polysaccharides (i.e., dietary fibers) are commonly classified as “soluble” or “insoluble” (in the GIT, i.e., an aqueous environment). However, there exists a wide range of solubility beyond these two extremes (poorly soluble, swollen gel-like networks, etc...) [49]. |
| Soluble (in water) β-glucan Pectins Gums Inulin Some hemicelluloses |
| Insoluble (in water) Cellulose LigninSome hemicelluloses | |||
| Viscosity | Internal friction of a liquid, or its tendency to resist flow |
| β-glucan Pectins Gums |
| Water-holding capacity | Ability of a fiber source to retain water within its matrix |
| Cellulose Lignin Some hemicelluloses (e.g., Arabinoxylans) |
| Fermentation | Breakdown of dietary fiber (including resistant starch and oligosaccharides), and some other undigested food components by bacteria in the large intestine. |
| Rapidly fermented Pectins β-glucans Arabinoxylans |
| Slowly fermented Resistant starch Cross-linked pectins Xylans Xyloglucans |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. https://doi.org/10.3390/foods9020201
Holland C, Ryden P, Edwards CH, Grundy MM-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods. 2020; 9(2):201. https://doi.org/10.3390/foods9020201
Chicago/Turabian StyleHolland, Claire, Peter Ryden, Cathrina H. Edwards, and Myriam M.-L. Grundy. 2020. "Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility" Foods 9, no. 2: 201. https://doi.org/10.3390/foods9020201
APA StyleHolland, C., Ryden, P., Edwards, C. H., & Grundy, M. M.-L. (2020). Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods, 9(2), 201. https://doi.org/10.3390/foods9020201





