Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture Preparation
2.2. Preparation of E. aerogenes and S. capitis in Phosphate Buffer and Marlin Meat Slurry
2.3. High Hydrostatic Pressure Treatment
2.4. Enumeration of HFB Surviving Cells and Decimal Reduction Time
2.5. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
3.1. Inactivation Kinetics of HHP Treatment on Histamine-Forming Bacteria in Phosphate Buffer
3.2. Inactivation Kinetics of HHP Treatment on Histamine-Forming Bacteria in Marlin Meat Slurry
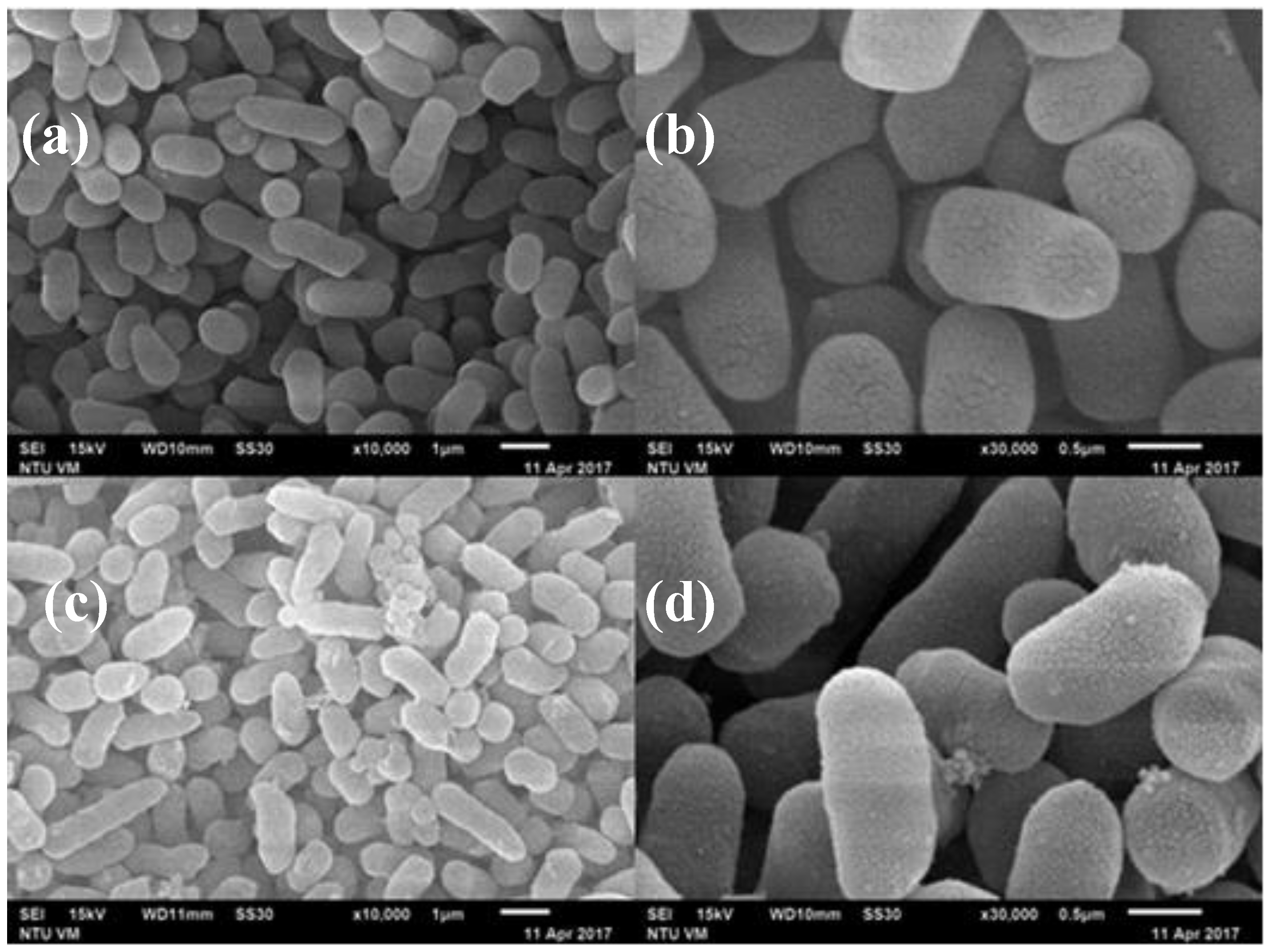
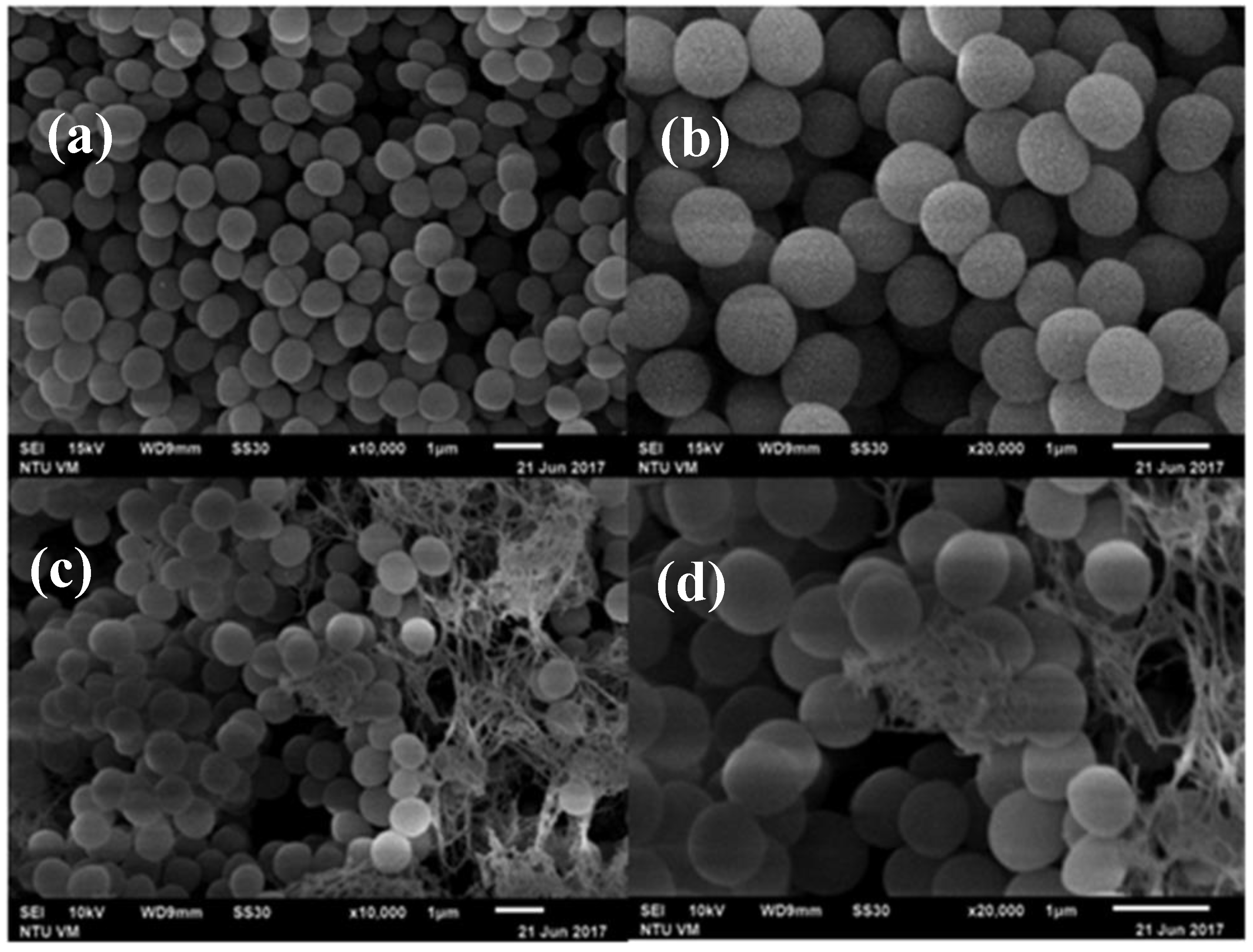
3.3. SEM Micrographs of Histamine-Forming Bacteria after Exposure to HHP Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Taylor, S.L. Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef]
- van Poelje, P.D.; Snell, E.E. Pyruvoyl-dependent enzymes. Annu. Rev. Biochem. 1990, 59, 29–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Price, R.J.; Morrissey, M.T.; Field, K.G.; Wei, C.I.; An, H. Occurrence of histamine-forming bacteria in albacore and histamine accumulation in muscle at ambient temperature. J. Food Sci. 2002, 67, 1515–1521. [Google Scholar] [CrossRef]
- Kung, H.F.; Lee, T.M.; Lin, C.S.; Hong, T.Y.; Tsai, Y.H. Polymerase chain reaction for the detection of histamine-producing bacteria isolated from Taiwanese foods. J. Food Drug Anal. 2012, 20, 74–82. [Google Scholar]
- Kim, S.H.; Wei, C.I.; Clemens, R.A.; An, H. Histamine accumulation in seafoods and its control to prevent outbreaks of scombroid poisoning. J. Aquat. Food Prod. Technol. 2004, 13, 81–100. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent advances in food processing using high hydrostatic pressure technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing -effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Phuasate, S.; Su, Y.C. Efficacy of low-temperature high hydrostatic pressure processing in inactivating Vibrio parahaemolyticus in culture suspension and oyster homogenate. Int. J. Food Microbiol. 2015, 196, 11–15. [Google Scholar] [CrossRef]
- Singh, A.; Ramaswamy, H. Effect of high pressure processing on color and textural properties of eggs. J. Food Res. 2013, 2, 11–24. [Google Scholar] [CrossRef]
- Christensen, A.; Hovda, M.B.; Rode, T.M. Quality changes in high pressure processed cod, salmon and mackerel during storage. Food Control. 2017, 72, 90–96. [Google Scholar] [CrossRef]
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C.; Marques, A.; Saraiva, J.A.; Nunes, M.L. Effect of high pressure processing in the quality of sea bass (Dicentrarchus labrax) fillets: Pressurization rate, pressure level and holding time. Innov. Food Sci. Emerg. Technol. 2014, 22, 31–39. [Google Scholar] [CrossRef]
- Ramaswamy, H.; Zaman, S.U.; Smith, J.P. High pressure destruction kinetics of Escherichia coli (O157:H7) and Listeria monocytogenes (Scott A) in a fish slurry. J. Food Eng. 2008, 87, 99–106. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Shyu, Y.T.; Yang, B.B. Inactivation and morphological damage of Vibrio parahaemolyticus treated with high hydrostatic pressure. Food Control 2013, 32, 348–353. [Google Scholar] [CrossRef]
- Huang, H.W.; Lung, H.M.; Yang, B.B.; Wang, C.Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Kung, H.F.; Lee, T.M.; Lin, G.T.; Hwang, D.F. Histamine related hygienic qualities and bacteria found in popular commercial scombroid fish fillets in Taiwan. J. Food Prot. 2004, 67, 407–412. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chuang, T.C.; Lin, H.C.; Huang, Y.R.; Lin, C.M.; Kung, H.F.; Tsai, Y.H. Histamine content and histamine-forming bacteria in dried milkfish (Chanos chanos) products. Food Chem. 2009, 114, 933–938. [Google Scholar] [CrossRef]
- Smelt, J.; Rijke, G. High pressure treatment as a tool for pasteurization of foods. In High Pressure and Biotechnology; Balny, C., Hayashi, R., Heremans, K., Masson, P., Eds.; Colloque INSERM/John Libbey Eurotex Ltd.: Montrouge, France, 1992; pp. 361–364. [Google Scholar]
- Patterson, M.F.; Quinn, M.; Simpson, R.; Gilmour, A. Effects of high pressure on vegetative pathogens. In High Pressure Processing of Foods; Ledward, D.A., Johnston, D.E., Earnshaw, R.G., Eds.; Nottingham University Press: Nottingham, UK, 1995; pp. 47–63. [Google Scholar]
- Furukawa, S.; Noma, S.; Shimoda, M.; Hayakawa, I. Effect of initial concentration of bacterial suspensions on their inactivation by high hydrostatic pressure. Int. J. Food Sci. Technol. 2002, 37, 573–577. [Google Scholar] [CrossRef]
- Dogan, C.; Erkmen, O. High pressure inactivation kinetics of Listeria monocytogenes inactivation in broth, milk, and peach and orange juices. J. Food Eng. 2004, 62, 47–52. [Google Scholar] [CrossRef]
- Tassou, C.; Galiatsatou, P.; Samaras, F.J.; Mallidis, C.G. Inactivation kinetics of a piezotolerant Staphylococcus aureus isolated from high-pressure-treated sliced ham by high pressure in buffer and in a ham model system: Evaluation in selective and non-selective medium. Innov. Food Sci. Emerg. Technol. 2007, 8, 478–484. [Google Scholar] [CrossRef]
- Alpas, H.; Kalchayanand, N.; Bozoglu, F.; Ray, B. Interactions of high hydrostatic pressure, pressurization temperature and pH on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. Int. J. Food Microbiol. 2000, 60, 33–42. [Google Scholar] [CrossRef]
- Hoover, D.G.; Metrick, C.; Papineau, A.M.; Farkas, D.F.; Knorr, D. Biological effects of high hydrostatic pressure on food microorganisms. Food Technol. 1989, 43, 99–107. [Google Scholar]
- Simpson, R.K.; Gilmour, A. The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J. Appl. Microbiol. 1997, 83, 181–188. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Culioli, J. Effects of high pressure on meat: A review. Meat Sci. 1997, 46, 211–236. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C.; Manitsa, C.; Mallidis, G. Modelling the effect of high pressure on the inactivation kinetics of a pressure-resistant strain of Pediococcus damnosus in phosphate buffer and gilt-head seabream (Sparus aurata). J. Appl. Microbiol. 2006, 102, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F. Microbiology of pressure-treated foods. J. Appl. Microbiol. 2005, 98, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Pilavtepe-Çelik, M.; Balaban, M.O.; Alpas, H.; Yousef, A.E. Image Analysis based quantification of bacterial volume change with high hydrostatic pressure. J. Food Sci. 2008, 73, 423–429. [Google Scholar] [CrossRef]
- Ritz, M.; Tholozan, J.L.; Federighi, M.; Pilet, M.F. Morphological and physiological characterization of Listeria monocytogenes subjected to high hydrostatic pressure. Appl. Environ. Microbiol. 2001, 67, 2240–2247. [Google Scholar] [CrossRef]
- Mackey, B.M.; Forestiere, K.; Isaacs, N.S.; Stenning, R.; Brooker, B. The effect of high hydrostatic pressure on Salmonella thompson and Listeria monocytogenes examined by electron microscopy. Lett. Appl. Microbiol. 1994, 19, 429–432. [Google Scholar] [CrossRef]
- Marx, G.; Moody, A.; Bermúdez-Aguirre, D. A comparative study on the structure of Saccharomyces cerevisiae under nonthermal technologies: High hydrostatic pressure, pulsed electric fields and thermo-sonication. Int. J. Food Microbiol. 2011, 151, 327–337. [Google Scholar] [CrossRef]
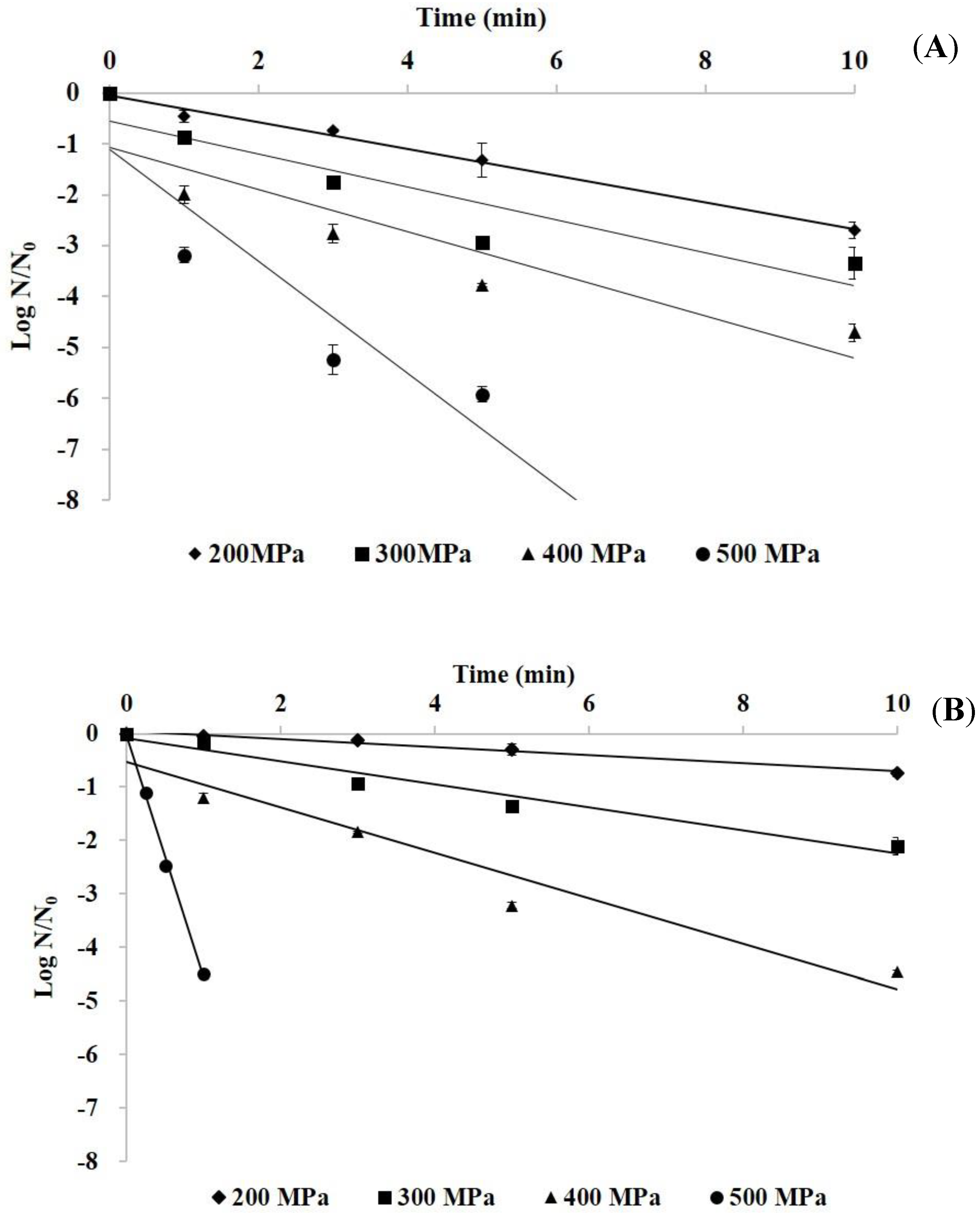
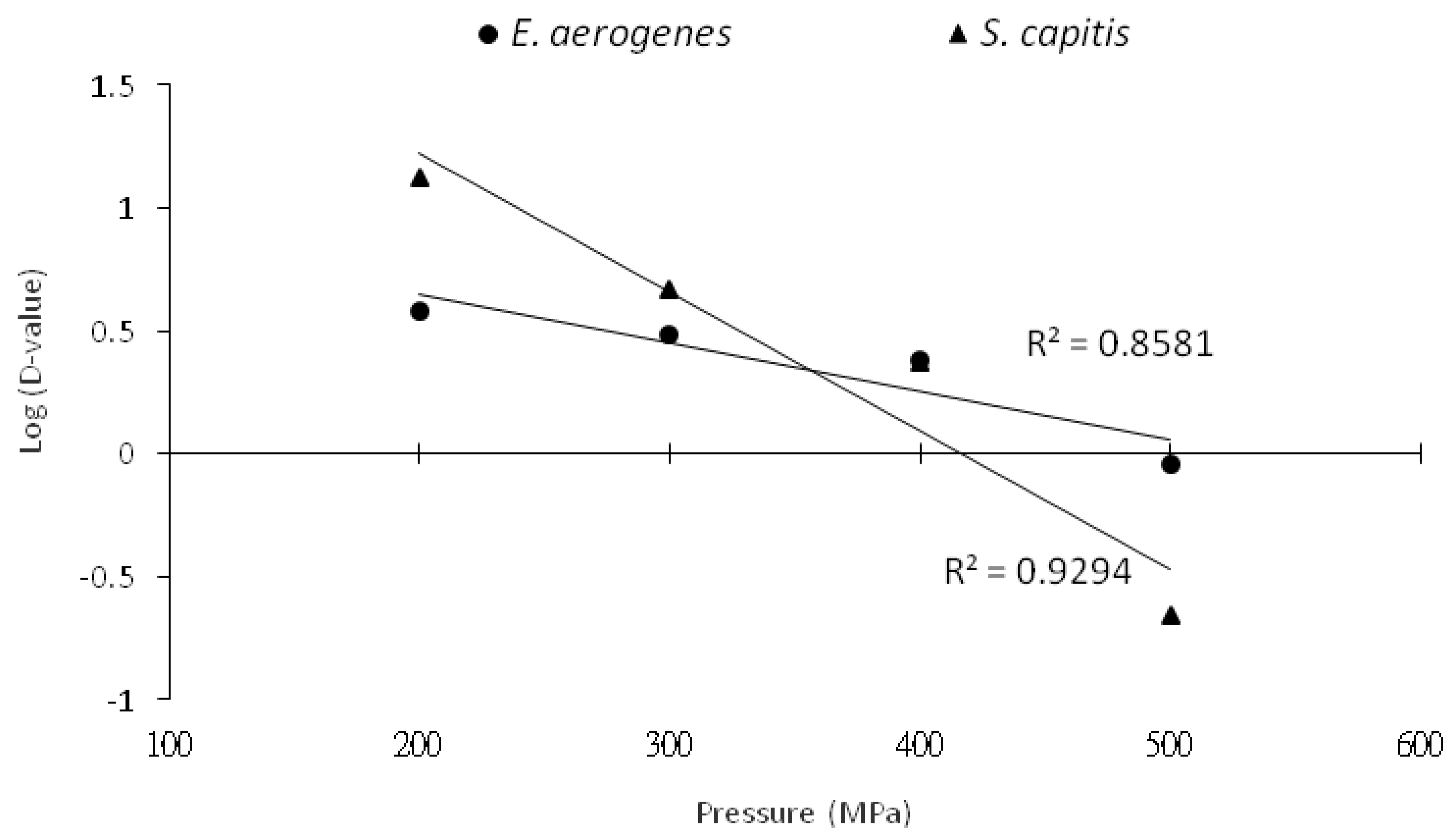
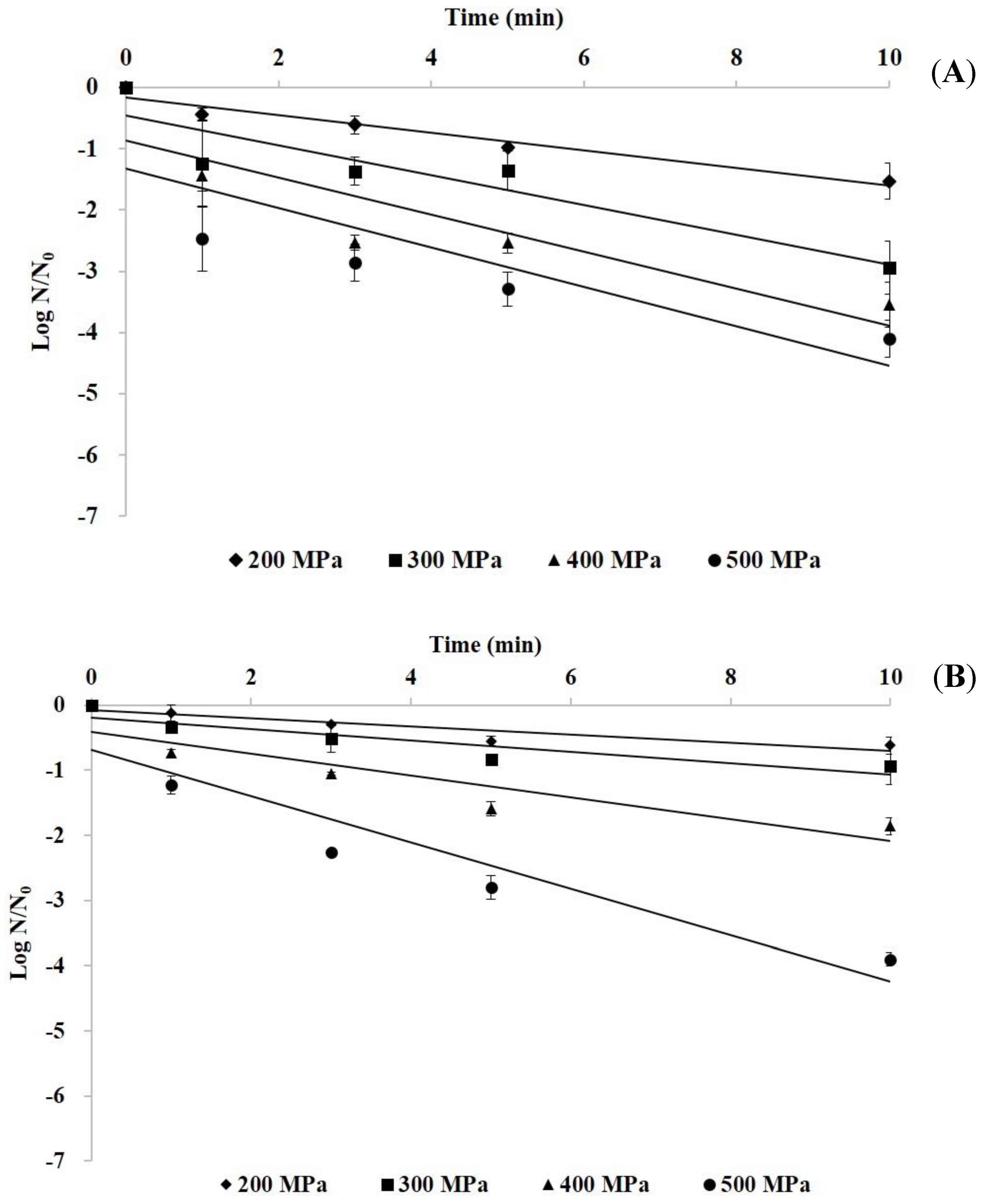
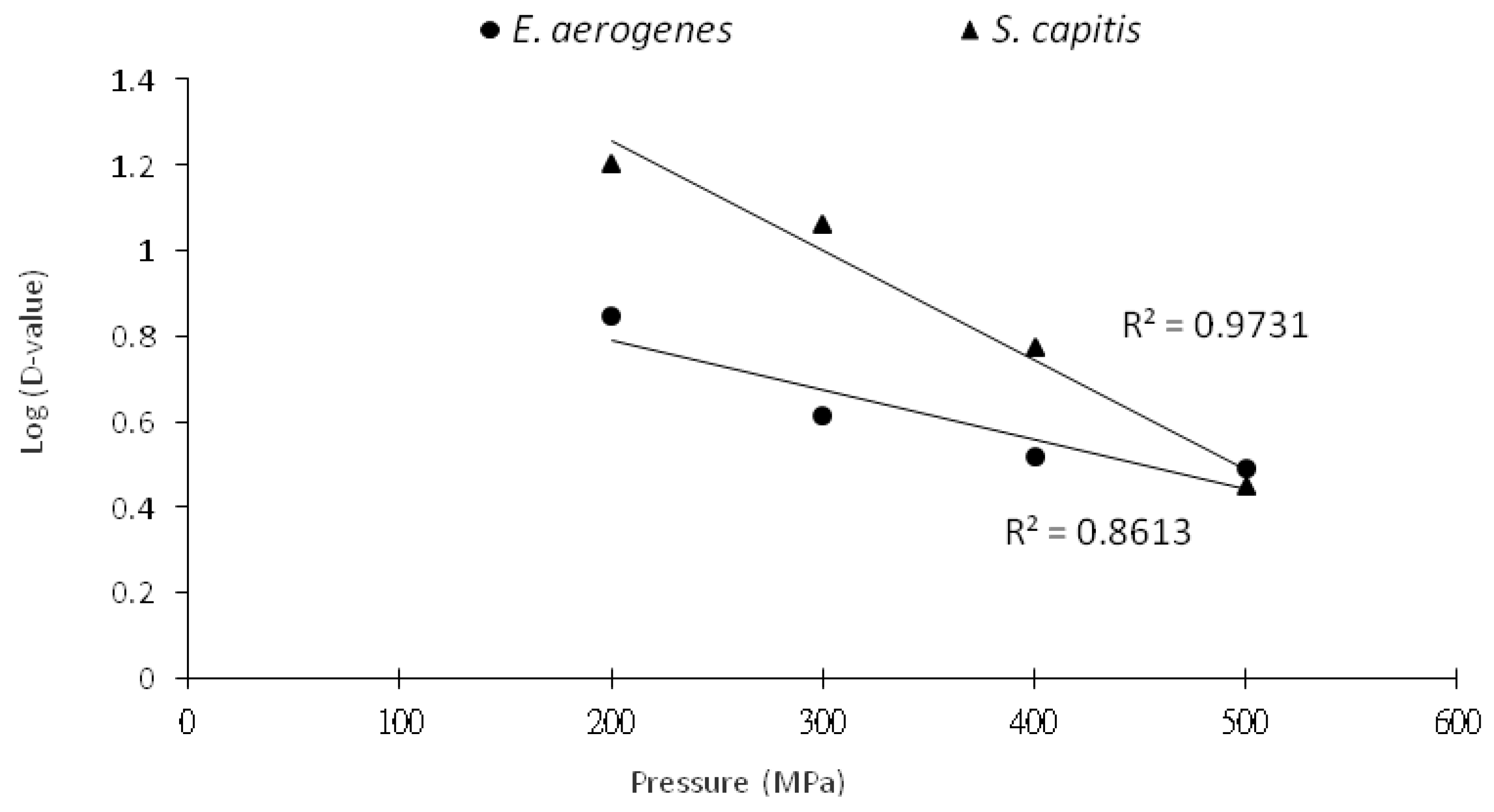
| Pressure (MPa) | Slope | D-Value (min) a | R2 |
|---|---|---|---|
| In phosphate buffer | |||
| E. aerogenes | |||
| 200 | −0.26 | 3.82 | 0.98 |
| 300 | −0.33 | 3.08 | 0.86 |
| 400 | −0.41 | 2.42 | 0.83 |
| 500 | −1.10 | 0.92 | 0.85 |
| S. capitis | |||
| 200 | −0.07 | 13.4 | 0.98 |
| 300 | −0.22 | 4.65 | 0.96 |
| 400 | −0.43 | 2.35 | 0.94 |
| 500 | −4.52 | 0.22 | 0.99 |
| In marlin meat slurry | |||
| E. aerogenes | |||
| 200 | −0.14 | 7.00 | 0.95 |
| 300 | −0.24 | 4.11 | 0.86 |
| 400 | −0.31 | 3.26 | 0.85 |
| 500 | −0.33 | 2.99 | 0.83 |
| S. capitis | |||
| 200 | −0.06 | 16.0 | 0.86 |
| 300 | −0.09 | 10.9 | 0.85 |
| 400 | −0.17 | 5.87 | 0.85 |
| 500 | −0.36 | 2.82 | 0.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Tsai, Y.-H.; Chen, S.-L.; Kung, H.-F.; Arakawa, O.; Wei, C.-I. Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure. Foods 2020, 9, 266. https://doi.org/10.3390/foods9030266
Lee Y-C, Tsai Y-H, Chen S-L, Kung H-F, Arakawa O, Wei C-I. Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure. Foods. 2020; 9(3):266. https://doi.org/10.3390/foods9030266
Chicago/Turabian StyleLee, Yi-Chen, Yung-Hsiang Tsai, Shao-Lan Chen, Hsien-Feng Kung, Osamu Arakawa, and Cheng-I Wei. 2020. "Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure" Foods 9, no. 3: 266. https://doi.org/10.3390/foods9030266
APA StyleLee, Y.-C., Tsai, Y.-H., Chen, S.-L., Kung, H.-F., Arakawa, O., & Wei, C.-I. (2020). Inactivation and Damage of Histamine-Forming Bacteria by Treatment with High Hydrostatic Pressure. Foods, 9(3), 266. https://doi.org/10.3390/foods9030266





