Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. RAW 264.7 Cell Culture
2.5. Measurement of RAW 264.7 Cell Viability
2.6. Measurement of NO Production in RAW 264.7 Cells
2.7. Western Blot Analysis
2.8. Determination of IL-6 and TNF-α Production
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Compounds
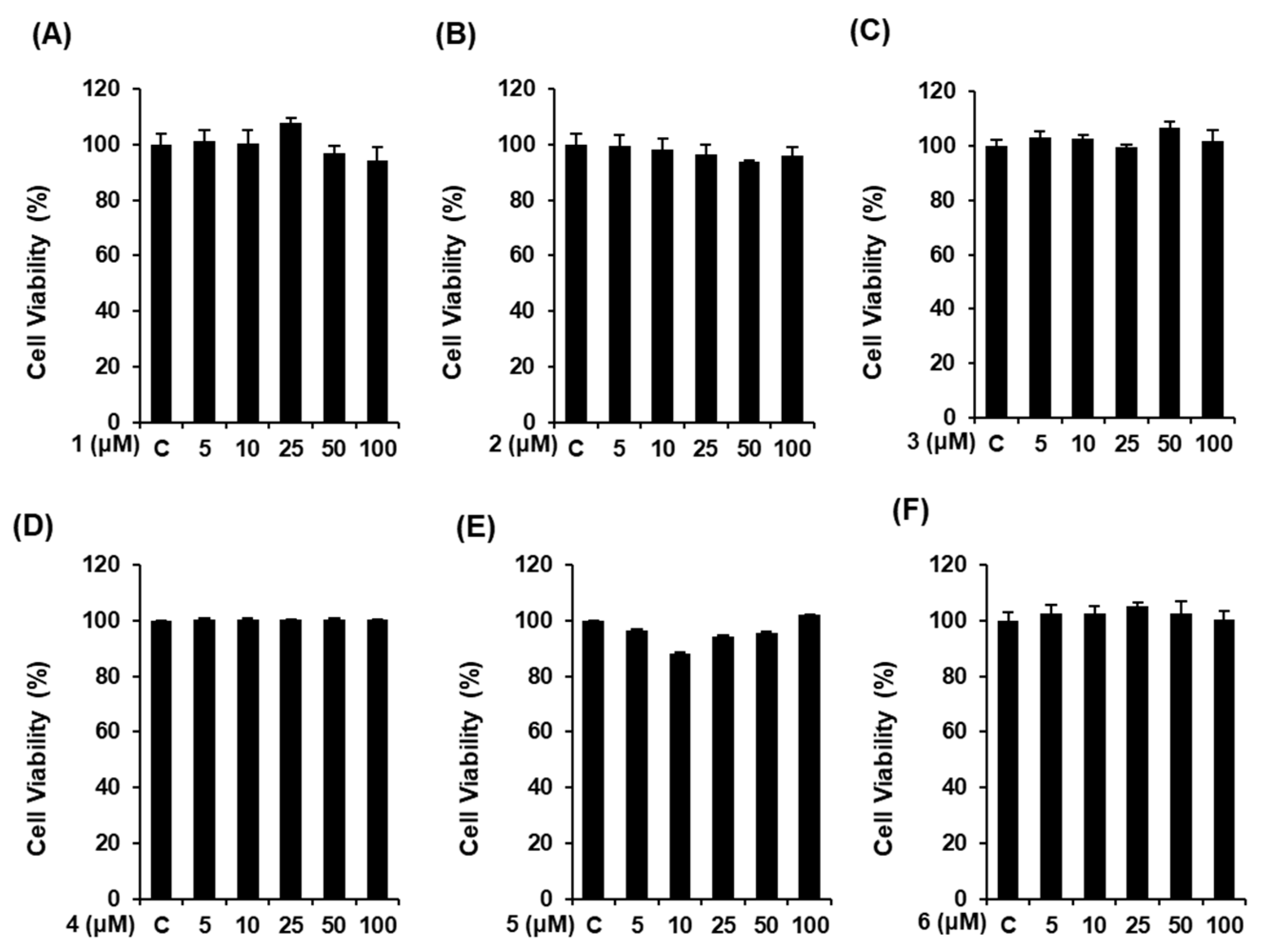
3.2. Effects of Compounds 1–6 on Cell Viability
3.3. Effects of Compounds 1–6 on NO Production
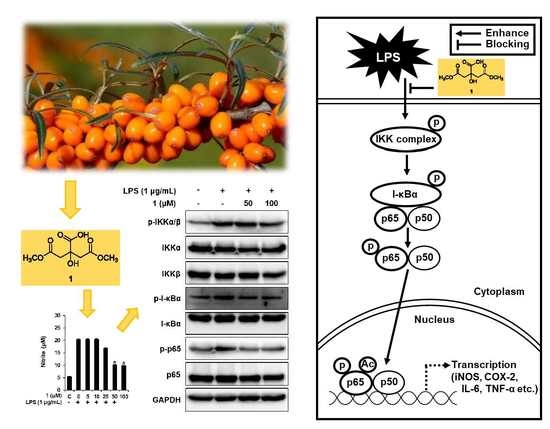
3.4. Compound 1 Downregulated IKKα/β, I-κBα, and NF-κB p65 in LPS-Stimulated RAW 264.7 Mouse Macrophages
3.5. Compound 1 downregulated iNOS and COX-2 expression in LPS-stimulated RAW 264.7 mouse macrophages
3.6. Compound 1 downregulated IL-6 and TNF-α production in LPS-stimulated RAW 264.7 mouse macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, I.; Khan, L.; Gul, A.; Ahmed, N.; Saleem, M. Comparative study of vitamin C contents in fruits and medicinal plants. J. Chem. Soc. Pak. 2008, 30, 406–409. [Google Scholar]
- Xu, X.; Gao, Y.; Liu, G.; Wang, Q.; Zhao, J. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae rhamnoides L.) oil using response surface methodology. LWT Food Sci. Technol. 2008, 41, 1223–1231. [Google Scholar] [CrossRef]
- Yang, B.R.; Kalimo, K.O.; Tahvonen, R.L.; Matilla, L.M.; Katajisto, J.K.; Kallio, H.P. Effect of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids patients with atopic dermatitis. J. Nutr. Biochem. 2000, 11, 338–340. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, Z.; Li, G.; Ouyang, J.; Suo, Y.; Wang, H. Isolation and structure determination of a mew flavone glycoside from seed residues of seabuckthorn (Hippophae rhamnoides L.). Nat. Prod. Res. 2018, 32, 892–897. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Rolnik, A.; Olas, B.; Stochmal, A.; Żuchowski, J. Anti-platelet properties of phenolic extracts from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules 2019, 24, 3620. [Google Scholar] [CrossRef]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) A. Nelson, extracts as virulence modulators-in vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1498. [Google Scholar] [CrossRef]
- Rösch, D.; Mügge, C.; Fogliano, V.; Kroh, L.W. Antioxidatn oligomeric proanthocyanidins from sea buckthorn (Hippophaë rhamnoides) pomace. J. Agric. Food Chem. 2004, 52, 6712–6718. [Google Scholar] [CrossRef]
- OuYang, J.; Zhou, W.N.; Li, G.; Wang, X.Y.; Ding, C.X.; Suo, Y.R.; Wang, H.L. Three New Alkaloids from Hippophae rhamnoides Linn. subsp. sinensis Rousi. Helv. Chim. Acta. 2015, 98, 1287–1291. [Google Scholar] [CrossRef]
- Gutzeit, D.; Wray, V.; Winterhalter, P.; Jerz, G. Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophaë rhamnoides L. ssp. rhamnoides) by high-speed counter-current chromatography. Chromatogrphia 2007, 65, 1–7. [Google Scholar]
- Chen, C.; Gao, W.; Cheng, L.; Shao, Y.; Kong, D.Y. Four new triterpenoid glycosides from the seed residue of Hippophae rhamnoides subsp sinensis. J. Asian Nat. Prod. Res. 2014, 16, 231–239. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Liu, P. Active components from sea buckthorn (Hippophae rhamnoides L.) regulate hepatic stellate cell activation and liver fibrogenesis. J. Agric. Food Chem. 2018, 66, 12257–12264. [Google Scholar] [CrossRef]
- Lau, T.A.; Bray, W.M.; Lokey, R.S. Macrophage Cytological Profiling and Anti-Inflammatory Drug Discovery. Assay Drug Dev. Techn. 2019, 17, 14–16. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta 1999, 1411, 401–414. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduc. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.-H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018, 41, 815–822. [Google Scholar] [PubMed]
- Yu, J.S.; Roh, H.-S.; Baek, K.-H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar]
- Yu, J.S.; Lee, D.; Lee, S.R.; Lee, J.W.; Choi, C.-I.; Jang, T.S.; Kang, K.S.; Kim, K.H. Chemical characterization of cytotoxic indole acetic acid derivative from Mulberry fruit (Morus alba L.) against human cervical cancer. Bioorg. Chem. 2018, 76, 28–36. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, D.U. Practical synthesis of novel citryl glycoside, the component of the fhizomes of Gastrodia elata. Bull. Korean Chem. Soc. 2008, 29, 2051–2053. [Google Scholar]
- Zhang, Y.H.; Yu, J.Q. Pd(II)-catalyzed hydroxylayion of arenes with 1 atm of O2 or air. J. Am. Chem. Soc. 2009, 131, 14654–14655. [Google Scholar] [CrossRef] [PubMed]
- Moco, S.; Tseng, L.H.; Spraul, M.; Chen, Z.; Vervoort, J. Building-up a comprehensive database of flavonoids based on nuclear magnetic resonance data. Chromatographia 2006, 64, 503–508. [Google Scholar] [CrossRef]
- Kai, H.; Baba, M.; Okuyama, T. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007, 55, 133–136. [Google Scholar] [CrossRef]
- Yamano, Y.; Ito, M. Synthesis of optically active vomifoliol and roseoside stereoisomers. Chem. Pharm. Bull. 2005, 53, 541–546. [Google Scholar] [CrossRef]
- McKenna, S.; Wright, C.J. Inhibiting IκBβ–NFκB signaling attenuates the expression of select pro-inflammatory genes. J. Cell Sci. 2015, 128, 2143–2155. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guan, H.; Liu, D.; Wu, X.; Fan, M.; Han, J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice. J. Med. Food. 2014, 17, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Choi, Y.S.; Lee, J.K.; Lee, B.J.; Kim, W.K.; Kang, H. Anti-Inflammatory Activity of Citric Acid-Treated Wheat Germ Extract in Lipopolysaccharide-Stimulated Macrophages. Nutrients 2017, 9, 730. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, H.J.; Han, J.S. Anti-inflammatory effects of calcium citrate in RAW 264.7cells via suppression of NF-κB activation. Environ. Toxicol. Pharmacol. 2015, 39, 27–34. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Zheng, J.; Dai, J.; Ou, W.; Xu, W.; Ai, Q.; Zhang, W.; Niu, J.; Mai, K.; et al. Citric acid mitigates soybean meal induced inflammatory response and tight junction disruption by altering TLR signal transduction in the intestine of turbot, Scophthalmus maximus L. Fish Shellfish Immunol. 2019, 92, 181–187. [Google Scholar] [CrossRef]
- Mohammed, M.M.D.; Kobayashi, N. Anti-Influenza a virus of a new oligosaccharide citric acid derivative isolated from Vigna angularis (ohwi et ohashi. var. Dainagon) seeds. J. Carbohyd. Chem. 2019, 38, 234–245. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, S.W.; Jin, S.A.; Ban, J.Y.; Hong, S.J.; Park, M.S. Protective effect of citric acid against hepatic ischemia reperfusion injury in Sprague-Dawley rats. Transplant. Proc. 2019, 51, 2823–2827. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.C.; Lee, D.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Kang, K.S.; Yamabe, N.; Kim, K.H. Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages. Foods 2020, 9, 269. https://doi.org/10.3390/foods9030269
Baek SC, Lee D, Jo MS, Lee KH, Lee YH, Kang KS, Yamabe N, Kim KH. Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages. Foods. 2020; 9(3):269. https://doi.org/10.3390/foods9030269
Chicago/Turabian StyleBaek, Su Cheol, Dahae Lee, Mun Seok Jo, Kwang Ho Lee, Yong Hoon Lee, Ki Sung Kang, Noriko Yamabe, and Ki Hyun Kim. 2020. "Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages" Foods 9, no. 3: 269. https://doi.org/10.3390/foods9030269
APA StyleBaek, S. C., Lee, D., Jo, M. S., Lee, K. H., Lee, Y. H., Kang, K. S., Yamabe, N., & Kim, K. H. (2020). Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages. Foods, 9(3), 269. https://doi.org/10.3390/foods9030269