Combined Treatments of High Hydrostatic Pressure and CO2 in Coho Salmon (Oncorhynchus kisutch): Effects on Enzyme Inactivation, Physicochemical Properties, and Microbial Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. High Hydrostatic Pressure and Carbon Dioxide (HHP + CO2)
2.3. Enzymatic Performance
2.3.1. Protease and Lipase Activity
2.3.2. Collagenase Activity
2.4. Physicochemical Properties
2.4.1. Total Volatile Basic Nitrogen (TVB-N), Trimethylamine (TMA), and Thiobarbituric Acid (TBA) Values
2.4.2. Textural Properties and Surface Color
2.5. Microbial Analysis and Shelf-Life Estimation
2.6. Statistical Analysis of Quality Parameters
3. Results and Discussion
3.1. Enzymatic Activities
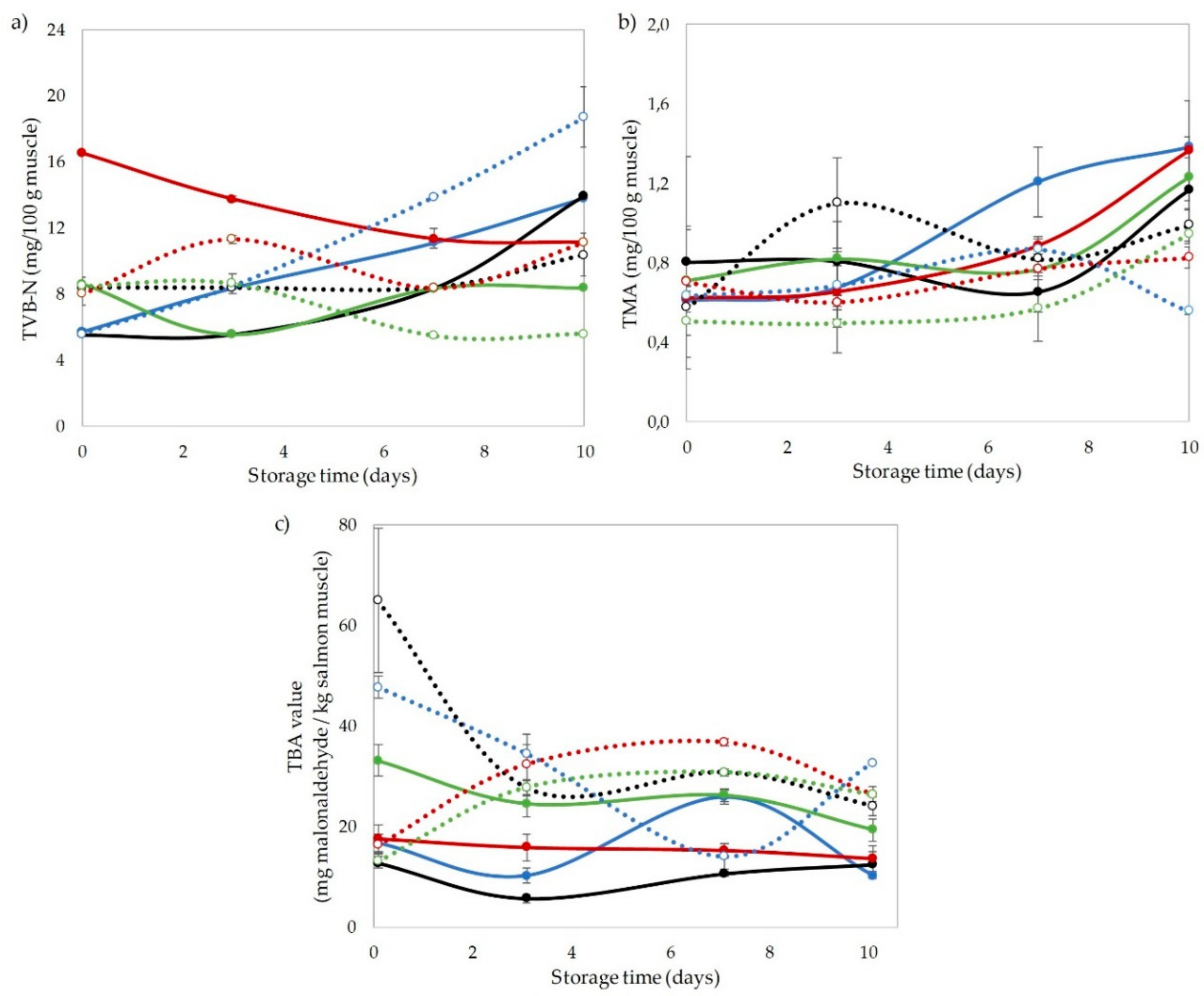
3.2. Chemical Indices
3.3. Effect of Carbon Dioxide (CO2) and High Hydrostatic Pressure (HHP) Treatment on Color and Texture of Coho Salmon
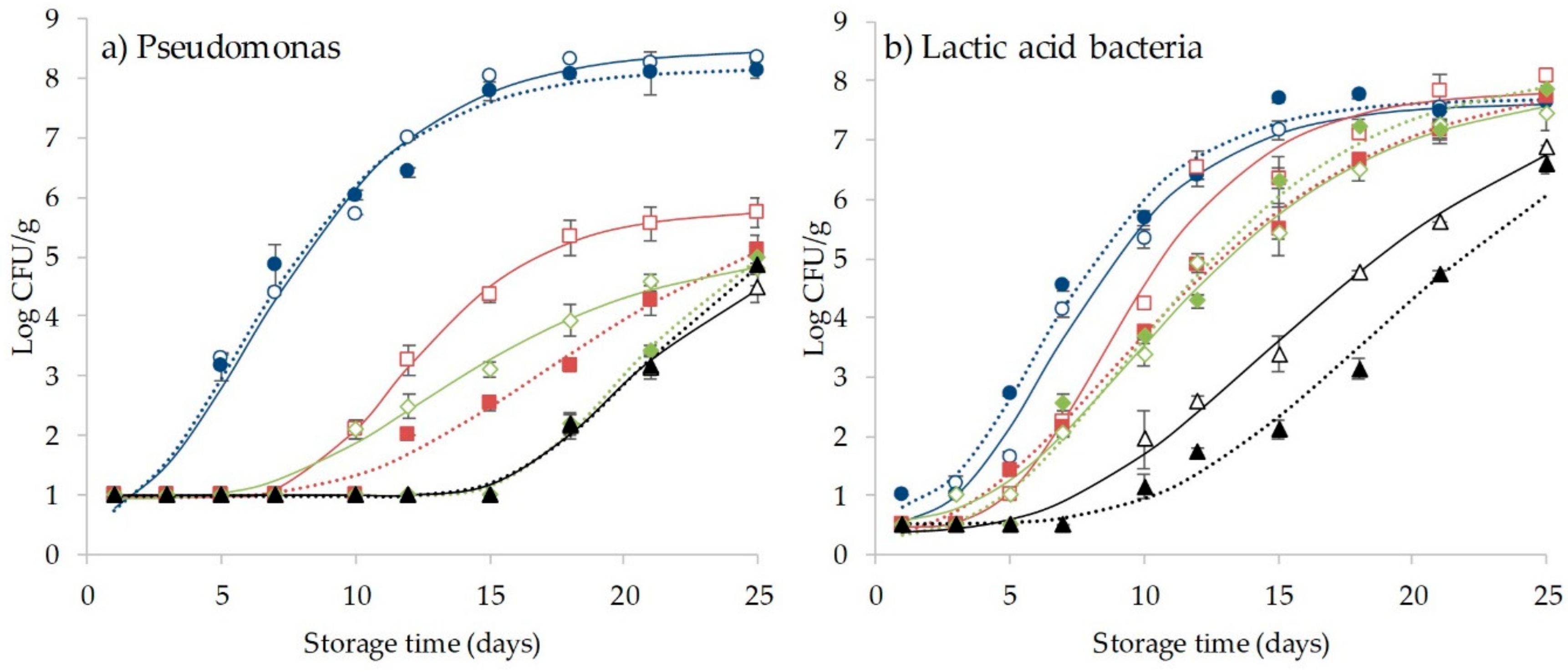
3.4. Effect of CO2 and HHP Treatment on Microbial Behavior and Shelf-Life Extension of Coho Salmon
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vinagre, J.; Rodríguez, A.; Larraín, M.A.; Aubourg, S.P. Chemical composition and quality loss during technological treatment in coho salmon (Oncorhynchus kisutch). Food Res. Int. 2011, 44, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, G.C.; Summers, G.; Corrigan, V.; Cumarasamy, S.; Dufour, J.P. Spoilage of king salmon (Oncorhynchus tshawytscha) fillets stored under different atmospheres. J. Food Sci. 2002, 67, 2362–2374. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Influence of packaging strategy on microbiological and biochemical changes in high-pressure-treated oysters (Crassostrea gigas). J. Sci. Food Agric. 2008, 88, 2713–2723. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Ge, L.; Xia, W.; Jiang, Q. The impact of collagen on softening of grass carp (Ctenopharyngodon idella) fillets stored under superchilled and ice storage. Int. J. Food Sci. Technol. 2015, 50, 2427–2435. [Google Scholar] [CrossRef]
- Hernández-Herrero, M.M.; Duflos, G.; Malle, P.; Bouquelet, S. Collagenase activity and protein hydrolysis as related to spoilage of iced cod (Gadus morhua). Food Res. Int. 2003, 36, 141–147. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Ge, L.; Zang, J.; Xia, W.; Jiang, Q. Inhibitory Effect of Edible Additives on Collagenase Activity and Softening of Chilled Grass Carp Fillets. J. Food Process. Preserv. 2017, 41, e12836. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and Its Control Using Protease Inhibitors in Fish and Fish Products: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Hultmann, L.; Phu, T.M.; Tobiassen, T.; Aas-Hansen, O.; Rustad, T. Effects of pre-slaughter stress on proteolytic enzyme activities and muscle quality of farmed Atlantic cod (Gadus morhua). Food Chem. 2012, 134, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed light treatments for food preservation. A review. Food Bioprocess Technol. 2010, 3, 13–23. [Google Scholar] [CrossRef]
- Ozen, B.F.; Floros, J.D. Effects of emerging food processing techniques on the packaging materials. Trends Food Sci. Technol. 2001, 12, 60–67. [Google Scholar] [CrossRef]
- Rodríguez, A.; Cruz, J.M.; Paseiro-Losada, P.; Aubourg, S.P. Effect of a Polyphenol-Vacuum Packaging on Lipid Deterioration During an 18-Month Frozen Storage of Coho Salmon (Oncorhynchus kisutch). Food Bioprocess Technol. 2012, 5, 2602–2611. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, F.A.; Neto, O.C.; dos Santos, L.M.R.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- Raso, J.; Barbosa-Cánovas, G.V. Nonthermal Preservation of Foods Using Combined Processing Techniques. Crit. Rev. Food Sci. Nutr. 2003, 43, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhou, L.; Xu, Z.; Zhang, Y.; Liao, X. Enzyme inactivation in food processing using high pressure carbon dioxide technology. Crit. Rev. Food Sci. Nutr. 2013, 53, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Guzman, F.; Illanes, A.; Wilson, L. Selective and eco-friendly synthesis of lipoaminoacid-based surfactants for food, using immobilized lipase and protease biocatalysts. Food Chem. 2018, 239, 189–195. [Google Scholar] [CrossRef]
- Apse, P.; Ellen, R.P.; Overall, C.M.; Zarb, G.A. Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: A comparison of sites in edentulous and partially edentulous patients. J. Periodontal Res. 1989, 24, 96–105. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Steinbrink, R. A Continuous Spectrophotometric Assay for Clostridium histolyticum Collagenase. Anal. Biochem. 1981, 365, 356–365. [Google Scholar] [CrossRef]
- Gallardo, J.M.; Perez-Martin, R.I.; Franco, J.M.; Aubourg, S.; Sotelo, C.G. Changes in volatile bases and trimethylamine oxide during the canning of albacore (Thunnus alalunga). Int. J. Food Sci. Technol. 1990, 25, 78–81. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 13th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Sørensen, G.; Storgaard Jørgensen, S. A critical examination of some experimental variables in the 2-thiobarbituric acid (TBA) test for lipid oxidation in meat products. Eur. Food Res. Technol. 1996, 202, 205–210. [Google Scholar]
- Briones-Labarca, V.; Perez-Won, M.; Zamarca, M.; Aguilera-Radic, J.M.; Tabilo-Munizaga, G. Effects of high hydrostatic pressure on microstructure, texture, colour and biochemical changes of red abalone (Haliotis rufecens) during cold storage time. Innov. Food Sci. Emerg. Technol. 2012, 13, 42–50. [Google Scholar] [CrossRef]
- Reyes, J.E.; Tabilo-Munizaga, G.; Pérez-Won, M.; Maluenda, D.; Roco, T. Effect of high hydrostatic pressure (HHP) treatments on microbiological shelf-life of chilled Chilean jack mackerel (Trachurus murphyi). Innov. Food Sci. Emerg. Technol. 2015, 29, 107–112. [Google Scholar] [CrossRef]
- EN ISO 15214. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C., 1st ed.; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Briones, L.S.; Reyes, J.E.; Tabilo-Munizaga, G.E.; Pérez-Won, M.O. Microbial shelf-life extension of chilled Coho salmon (Oncorhynchus kisutch) and abalone (Haliotis rufescens) by high hydrostatic pressure treatment. Food Control 2010, 21, 1530–1535. [Google Scholar] [CrossRef]
- EN ISO 7937. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Clostridium Perfringens—Colony-Count Technique, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. A novel approach for calculating shelf life of minimally processed vegetables. Int. J. Food Microbiol. 2006, 106, 69–73. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H. Effect of high pressure (HP) on the quality and shelf life of red mullet (Mullus surmelutus). Innov. Food Sci. Emerg. Technol. 2010, 11, 259–264. [Google Scholar] [CrossRef]
- Karim, N.U.; Kennedy, T.; Linton, M.; Watson, S.; Gault, N.; Patterson, M.F. Effect of high pressure processing on the quality of herring (Clupea harengus) and haddock (Melanogrammus aeglefinus) stored on ice. Food Control 2011, 22, 476–484. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov. Food Sci. Emerg. Technol. 2011, 12, 104–110. [Google Scholar] [CrossRef]
- Balaban, M.O.; Arreola, A.G.; Marshall, M.; Peplow, A.; Wei, C.I.; Cornell, J. Inactivation of Pectinesterase in Orange Juice by Supercritical Carbon Dioxide. J. Food Sci. 1991, 56, 743–746. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Bertucco, A. Non-Thermal Bacteria Inactivation with Dense CO2. Biotechnol. Bioeng. 2003, 84, 627–638. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Patterson, M.F.; Piggott, J.R. Effects of high-pressure processing on proteolytic enzymes and proteins in cold-smoked salmon during refrigerated storage. Food Chem. 2005, 90, 541–548. [Google Scholar] [CrossRef]
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C.; Marques, A.; Saraiva, J.A.; Nunes, M.L. Changes of enzymes activity and protein profiles caused by high-pressure processing in sea bass (dicentrarchus labrax) fillets. J. Agric. Food Chem. 2013, 61, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C.; Marques, A.; Alexandre, J.; Leonor, M. Effect of high pressure processing in the quality of sea bass (Dicentrarchus labrax) fillets: Pressurization rate, pressure level and holding time. Innov. Food Sci. Emerg. Technol. 2014, 22, 31–39. [Google Scholar] [CrossRef]
- Ishikawa, H.; Shimoda, M.; Yonekura, A.; Osajima, Y. Inactivation of Enzymes and Decomposition of α-Helix Structure by Supercritical Carbon Dioxide Microbubble Method. J. Agric. Food Chem. 1996, 44, 2646–2649. [Google Scholar] [CrossRef]
- Duong, T.; Balaban, M. Optimisation of the process parameters of combined high hydrostatic pressure and dense phase carbon dioxide on enzyme inactivation in feijoa (Acca sellowiana) puree using response surface methodology. Innov. Food Sci. Emerg. Technol. 2014, 26, 93–101. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, Y.; Yu, J.; Ling, J.; Shang, H.; Liu, Z. The Combined Efficacy of Superchilling and High CO 2 Modified Atmosphere Packaging on Shelf Life and Quality of Swimming Crab (Portunus trituberculatus). J. Aquat. Food Prod. Technol. 2017, 26, 655–664. [Google Scholar] [CrossRef]
- Amanatidou, A.; Schlüter, O.; Lemkau, K.; Gorris, L.G.M.; Smid, E.J.; Knorr, D. Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic almon. Innov. Food Sci. Emerg. Technol. 2000, 1, 87–98. [Google Scholar] [CrossRef]
- Rode, T.M.; Hovda, M.B. High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control 2016, 70, 242–248. [Google Scholar] [CrossRef]
- Chéret, R.; Chapleau, N.; Delbarre-Ladrat, C.; Verrez-Bagnis, V.; de Lamballerie, M. Effects of High Pressure on Texture and Microstructure of Sea Bass (Dicentrarchus labrax L.) Fillets. J. Food Sci. 2005, 70, e477–e483. [Google Scholar] [CrossRef] [Green Version]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Ribeiro Junior, E.E.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125. [Google Scholar] [CrossRef]
- Ottestad, S.; Sørheim, O.; Heia, K.; Skaret, J.; Wold, J.P. Effects of storage atmosphere and heme state on the color and visible reflectance spectra of salmon (Salmo salar) fillets. J. Agric. Food Chem. 2011, 59, 7825–7831. [Google Scholar] [CrossRef] [PubMed]
- Delbarre-Ladrat, C.; Chéret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in postmortem aging in fish: Understanding of proteolysis and disorganization of the myofibrillar structure. Crit. Rev. Food Sci. Nutr. 2006, 46, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Ayala, M.D.; Abdel, I.; Santaella, M.; Martínez, C.; Periago, M.J.; Gil, F.; Blanco, A.; Albors, O.L. Muscle tissue structural changes and texture development in sea bream, Sparus aurata L., during post-mortem storage. LWT Food Sci. Technol. 2010, 43, 465–475. [Google Scholar] [CrossRef]
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-life extension on fillets of Atlantic Salmon (Salmo salar) using natural additives, superchilling and modified atmosphere packaging. Food Control 2009, 20, 1036–1042. [Google Scholar] [CrossRef]
- Aponte, M.; Anastasio, A.; Marrone, R.; Mercogliano, R.; Peruzy, M.F.; Murru, N. Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT 2018, 93, 412–419. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Tabilo-Munizaga, G.; Reyes, J.E.; Rodríguez, A.; Pérez-Won, M. Effect of high-pressure treatment on microbial activity and lipid oxidation in chilled coho salmon. Eur. J. Lipid Sci. Technol. 2010, 112, 362–372. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Leiva-Portilla, D.; Perez-Won, M.; Tabilo-Munizaga, G.; Aubourg, S. Effects of High Pressure Treatment on Physicochemical Quality of Pre- and Post-Rigor Palm Ruff (Seriolella Violacea) Fillets. J. Aquat. Food Prod. Technol. 2018, 27, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Corbo, M.R.; Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Microbiological and sensorial quality assessment of ready-to-cook seafood products packaged under modified atmosphere. J. Food Sci. 2009, 74, M473–M478. [Google Scholar] [CrossRef]
- Gokoglu, N. Innovations in Seafood Packaging Technologies: A Review. Food Rev. Int. 2019, 1–27. [Google Scholar] [CrossRef]
| Treatment (MPa/CO2) | Storage Time (Days) | ||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 10 | ||
| 0/0 | 0.397 ± 0.002 fA | 0.241 ± 0.001 aB | 0.231 ± 0.011 abB | 0.364 ± 0.006 bC | |
| Protease activity (IU) | 0/50 | 0.280 ± 0.004 cA | 0.124 ± 0.004 bB | 0.439 ± 0.006 cC | 0.217 ± 0.004 aD |
| 0/70 | 0.194 ± 0.007 bA | 0.414 ± 0.019 cB | 0.281 ± 0.015 dC | 0.369 ± 0.030 bB | |
| 0/100 | 0.341 ± 0.003 eA | 0.179 ± 0.003 dB | 0.529 ± 0.035 eC | 0.200 ± 0.002 aB | |
| 150/0 | 0.389 ± 0.014 fA | 0.212 ± 0.001 eB | 0.354 ± 0.002 fC | 0.389 ± 0.011 bA | |
| 150/50 | 0.317 ± 0.008 dA | 0.111 ± 0.004 bB | 0.248 ± 0.008 adA | 0.756 ± 0.103 dC | |
| 150/70 | 0.389 ± 0.006 fA | 0.121 ± 0.004 bB | 0.202 ± 0.003 bgC | 0.341 ± 0.001 bD | |
| 150/100 | 0.163 ± 0.004 aA | 0.258 ± 0.013 aB | 0.168 ± 0.005 gA | 0.643 ± 0.013 cD | |
| Lipase activity (IU) | 0/0 | 0.379 ± 0.007 aA | 0.428 ± 0.006 aB | 0.296 ± 0.002 deC | 0.314 ± 0.013 dC |
| 0/50 | 0.279 ± 0.003 bA | 0.382 ± 0.002 aB | 0.301 ± 0.012 cdA | 0.270 ± 0.028 cA | |
| 0/70 | 0.246 ± 0.005 cA | 0.280 ± 0.002 bcB | 0.280 ± 0.002 eB | 0.271 ± 0.015 cB | |
| 0/100 | 0.390 ± 0.001 dA | 0.419 ± 0.058 aA | 0.219 ± 0.002 fB | 0.186 ± 0.003 aB | |
| 150/0 | 0.290 ± 0.006 eA | 0.290 ± 0.005 bA | 0.370 ± 0.007 aB | 0.207 ± 0.006 aC | |
| 150/50 | 0.607 ± 0.005 fA | 0.237 ± 0.227 bcB | 0.289 ± 0.001 deC | 0.233 ± 0.003 bB | |
| 150/70 | 0.555 ± 0.005 gA | 0.227 ± 0.014 cB | 0.315 ± 0.006 cC | 0.250 ± 0.019 bcB | |
| 150/100 | 0.408 ± 0.008 hA | 0.399 ± 0.014 aA | 0.335 ± 0.006 bB | 0.402 ± 0.010 eA | |
| Collagenase activity (IU) | 0/0 | 0.000 A | 1.675 ± 0.066 aB | 1.774 ± 0.068 aB | 2.458 ± 0.068 eD |
| 0/50 | 0.000 A | 0.531 ± 0.015 deB | 0.843 ± 0.074 bC | 0.781 ± 0.053 dC | |
| 0/70 | 0.000 A | 0.543 ± 0.032 cdeB | 1.966 ± 0.004 cC | 0.421 ± 0.006 bD | |
| 0/100 | 0.000 A | 1.198 ± 0.022 bB | 0.200 ± 0.019 fC | 0.229 ± 0.021 aC | |
| 150/0 | 0.000 A | 0.691 ± 0.091 cB | 0.476 ± 0.016 deC | 0.266 ± 0.019 aD | |
| 150/50 | 0.000 A | 0.307 ± 0.023 fB | 0.551 ± 0.042 dC | 0.411 ± 0.022 bcBC | |
| 150/70 | 0.000 A | 0.464 ± 0.002 eBC | 0.396 ± 0.039 eB | 0.543 ± 0.061 cC | |
| 150/100 | 0.000 A | 0.664 ± 0.016 cdB | 0.634 ± 0.009 dB | 0.210 ± 0.023 aC | |
| Treatment (MPa/CO2) | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 3 | 7 | 10 | |
| 0/0 | 6.05 ± 0.10 aA | 6.22 ± 0.02 aB | 6.19 ± 0.02 aAB | 6.20 ± 0.01 abB |
| 0/50 | 6.16 ± 0.02 abA | 6.16 ± 0.03 bA | 6.15 ± 0.01 bA | 6.08 ± 0.15 bcA |
| 0/70 | 6.16 ± 0.02 abAB | 6.20 ± 0.03 abA | 6.07 ± 0.02 cB | 5.90 ± 0.09 dC |
| 0/100 | 6.13 ± 0.03 abA | 6.20 ± 0.01 abB | 6.14 ± 0.01 bA | 6.00 ± 0.02 cdC |
| 150/0 | 6.35 ± 0.03 bA | 6.21 ± 0.01 abB | 6.24 ± 0.01 dB | 6.11 ± 0.01 abcC |
| 150/50 | 6.15 ± 0.02 abA | 6.21 ± 0.01 abB | 6.20 ± 0.01 aB | 6.28 ± 0.02 aC |
| 150/70 | 6.30 ± 0.01 bcA | 6.21 ± 0.03 abB | 6.22 ± 0.02 adB | 6.24 ± 0.01 abB |
| 150/100 | 6.41 ± 0.13 cA | 6.22 ± 0.01 aB | 6.28 ± 0.02 eAB | 6.17 ± 0.02 abB |
| Treatment (MPa/CO2) | Color Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | |||||
| Day 0 | Day 10 | Day 0 | Day 10 | Day 0 | Day 10 | Day 0 | Day 10 | |
| 0/0 | 46.0 ± 0.0 aA | 49.6 ± 0.3 aB | 31.7 ± 0.1 aA | 23.3 ± 0.5 aB | 33.8 ± 0.1 aA | 27.2 ± 0.1 aB | 0.0 aA | 11.3 ± 0.3 aB |
| 0/50 | 46.1 ± 0.2 aA | 48.5 ± 0.1 Bb | 33.4 ± 0.2 abA | 33.4 ± 0.4 bcA | 36.2 ± 0.6 bA | 32.2 ± 0.6 bcB | 3.8 ± 0.3 bcA | 3.4 ± 0.3 dA |
| 0/70 | 48.3 ± 0.3 bA | 48.0 ± 0.0 cA | 33.8 ± 0.6 bA | 32.3 ± 0.1 cB | 34.2 ± 0.6 abA | 31.9 ± 0.1 bB | 3.3 ± 0.2 bA | 3.2 ± 0.3 dA |
| 0/100 | 50.6 ± 0.0 cA | 48.5 ± 0.2 bB | 38.1 ± 0.2 cA | 33.2 ± 0.2 bcdB | 33.0 ± 0.1 aA | 34.0 ± 2.3 bcdA | 4.7 ± 0.0 cdA | 3.4 ± 0.4 dB |
| 150/0 | 59.7 ± 0.3 dA | 53.1 ± 0.1 dB | 28.3 ± 1.7 dA | 36.8 ± 0.1 eB | 28.6 ± 1.7 cA | 34.6 ± 0.4 cdB | 15.2 ± 0.7 fA | 9.9 ± 1.2 abB |
| 150/50 | 50.1 ± 0.1 cA | 51.5 ± 0.2 cB | 32.3 ± 0.2 abA | 33.9 ± 0.1 bdB | 32.1 ± 0.1 adA | 33.9 ± 0.4 bcdB | 4.4 ± 0.1 cdA | 5.8 ± 0.2 cB |
| 150/70 | 52.7 ± 0.1 eA | 52.3 ± 0.1 eA | 31.7 ± 0.5 aA | 29.4 ± 0.4 fB | 30.5 ± 0.7 cdA | 35.0 ± 0.6 dB | 7.5 ± 0.6 eA | 6.7 ± 0.2 cA |
| 150/100 | 49.2 ± 0.1 fA | 55.2 ± 0.2 fB | 28.8 ± 0.4 dA | 32.5 ± 0.6 cdB | 36.3 ± 0.5 bA | 32.7 ± 0.3 bcdB | 5.4 ± 0.2 dA | 9.6 ± 0.4 bB |
| Treatment (MPa/CO2) | Texture Parameters | |||||
|---|---|---|---|---|---|---|
| Hardness (N) | Springiness (cm) | Cohesiveness | ||||
| Day 0 | Day 10 | Day 0 | Day 10 | Day 0 | Day 10 | |
| 0/0 | 12.92 ± 2.47 abA | 7.57 ± 1.03 abB | 0.57 ± 0.07 abcA | 0.67 ± 0.05 aB | 0.41 ± 0.02 aA | 0.47 ± 0.04 abB |
| 0/50 | 11.53 ± 3.22 abA | 6.90 ± 1.57 abB | 0.64 ± 0.04 aA | 0.65 ± 0.04 aA | 0.52 ± 0.04 bA | 0.42 ± 0.07 aB |
| 0/70 | 13.45 ± 1.78 aA | 8.22 ± 1.86 abB | 0.62 ± 0.00 abA | 0.67 ± 0.03 aB | 0.44 ± 0.04 aA | 0.50 ± 0.08 abA |
| 0/100 | 14.39 ± 3.51 abA | 7.22 ± 0.99 abB | 0.58 ± 0.01 bcA | 0.61 ± 0.04 aA | 0.42 ± 0.09 aA | 0.48 ± 0.10 abA |
| 150/0 | 11.39 ± 1.32 abA | 8.04 ± 1.19 abB | 0.51 ± 0.03 cA | 0.59 ± 0.08 aB | 0.40 ± 0.04 aA | 0.50 ± 0.07 abB |
| 150/50 | 10.18 ± 3.21 abA | 6.63 ± 1.85 bB | 0.50 ± 0.02 cA | 0.58 ± 0.05 aB | 0.51 ± 0.06 aA | 0.55 ± 0.10 bB |
| 150/70 | 14.84 ± 1.18 bA | 5.36 ± 1.10 bB | 0.50 ± 0.05 cA | 0.60 ± 0.04 aB | 0.35 ± 0.09 aA | 0.47 ± 0.04 abB |
| 150/100 | 14.07 ± 3.45 abA | 10.54 ± 4.80 aA | 0.46 ± 0.05 dA | 0.60 ± 0.02 aB | 0.25 ± 0.05 cA | 0.49 ± 0.04 abB |
| Treatment (MPa/CO2) | Microbial Groups | |||
|---|---|---|---|---|
| Aerobic Mesophilic Microorganisms | Aerobic Psychrophilic Microorganisms | Lactic Acid Bacteria | Pseudomonas spp. | |
| 0/0 | 1.69 ± 0.09 ab | 1.67 ± 0.06 a | < 1.00 a | < 2.00 a |
| 0/50 | 1.46 ± 0.15 ac | 1.42 ± 0.10 a | < 1.00 a | < 2.00 a |
| 0/70 | 1.56 ± 0.07 abc | 1.42 ± 0.10 a | < 1.00 a | < 2.00 a |
| 0/100 | 1.75 ± 0.05 ab | 1.46 ± 0.15 a | < 1.00 a | < 2.00 a |
| 150/0 | 1.76 ± 0.06 b | 1.65 ± 0.16 a | 1.00 ± 0.00 a | < 2.00 a |
| 150/50 | 1.46 ± 0.15 ac | < 1.00 b | < 1.00 a | < 2.00 a |
| 150/70 | 1.26 ± 0.24 c | < 1.00 b | < 1.00 a | < 2.00 a |
| 150/100 | 1.36 ± 0.10 c | < 1.00 b | < 1.00 a | < 2.00 a |
| Treatment (MPa/CO2) | Kinetic Parameters | |||||
|---|---|---|---|---|---|---|
| Aerobic Mesophilic | Aerobic Psychrophilic | |||||
| λ (Days) | μmax (1/Days) | SL (Days) | λ (Days) | μmax (1/Days) | SL (Days) | |
| 0/0 | 2.33 ± 1.25 a | 0.63 ± 0.07 a | 9.72 ± 1.04 a | 2.30 ± 1.48 ab | 0.68 ± 0.10 a | 9.48 ± 1.35 a |
| 0/50 | 5.10 ± 1.32 bc | 0.60 ± 0.09 ab | 13.66 ± 1.51 b | 4.79 ± 0.69 ab | 0.54 ± 0.04 abc | 14.05 ± 0.78 b |
| 0/70 | 5.97 ± 1.38 c | 0.48 ± 0.07 abc | 15.87 ± 1.61 b | 4.72 ± 1.84 ab | 0.42 ± 0.05 cd | 16.36 ± 1.51 b |
| 0/100 | 10.46 ± 0.66 d | 0.35 ± 0.02 c | 23.19 ± 0.93 c | 10.31 ± 1.15 c | 0.37 ± 0.05 cd | 22.14 ± 1.63 c |
| 150/0 | 2.47 ± 1.32 ab | 0.63 ± 0.09 a | 9.73 ± 1.22 a | 1.95 ± 1.77 a | 0.62 ± 0.09 ab | 9.63 ± 1.35 a |
| 150/50 | 4.99 ± 1.43 abc | 0.46 ± 0.06 bc | 16.01 ± 1.69 b | 3.02 ± 0.98 ab | 0.51 ± 0.03 bcd | 15.29 ± 0.89 b |
| 150/70 | 4.90 ± 1.02 abc | 0.44 ± 0.04 c | 16.71 ± 1.05 b | 5.21 ± 1.20 b | 0.55 ± 0.05 abc | 16.26 ± 1.53 b |
| 150/100 | 9.72 ± 1.04 d | 0.43 ± 0.06 c | 22.72 ± 2.04 c | 9.11 ± 1.15 c | 0.43 ± 0.05 cd | 22.40 ± 1.98 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Won, M.; Lemus-Mondaca, R.; Herrera-Lavados, C.; Reyes, J.E.; Roco, T.; Palma-Acevedo, A.; Tabilo-Munizaga, G.; Aubourg, S.P. Combined Treatments of High Hydrostatic Pressure and CO2 in Coho Salmon (Oncorhynchus kisutch): Effects on Enzyme Inactivation, Physicochemical Properties, and Microbial Shelf Life. Foods 2020, 9, 273. https://doi.org/10.3390/foods9030273
Perez-Won M, Lemus-Mondaca R, Herrera-Lavados C, Reyes JE, Roco T, Palma-Acevedo A, Tabilo-Munizaga G, Aubourg SP. Combined Treatments of High Hydrostatic Pressure and CO2 in Coho Salmon (Oncorhynchus kisutch): Effects on Enzyme Inactivation, Physicochemical Properties, and Microbial Shelf Life. Foods. 2020; 9(3):273. https://doi.org/10.3390/foods9030273
Chicago/Turabian StylePerez-Won, Mario, Roberto Lemus-Mondaca, Carolina Herrera-Lavados, Juan E. Reyes, Teresa Roco, Anais Palma-Acevedo, Gipsy Tabilo-Munizaga, and Santiago P. Aubourg. 2020. "Combined Treatments of High Hydrostatic Pressure and CO2 in Coho Salmon (Oncorhynchus kisutch): Effects on Enzyme Inactivation, Physicochemical Properties, and Microbial Shelf Life" Foods 9, no. 3: 273. https://doi.org/10.3390/foods9030273





