In Vitro Bioaccessibility of Ripe Table Olive Mineral Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Experimental Design
2.2. Cleaning of the Material
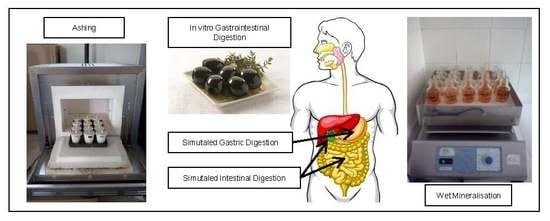
2.3. In Vitro Digestion of Olives
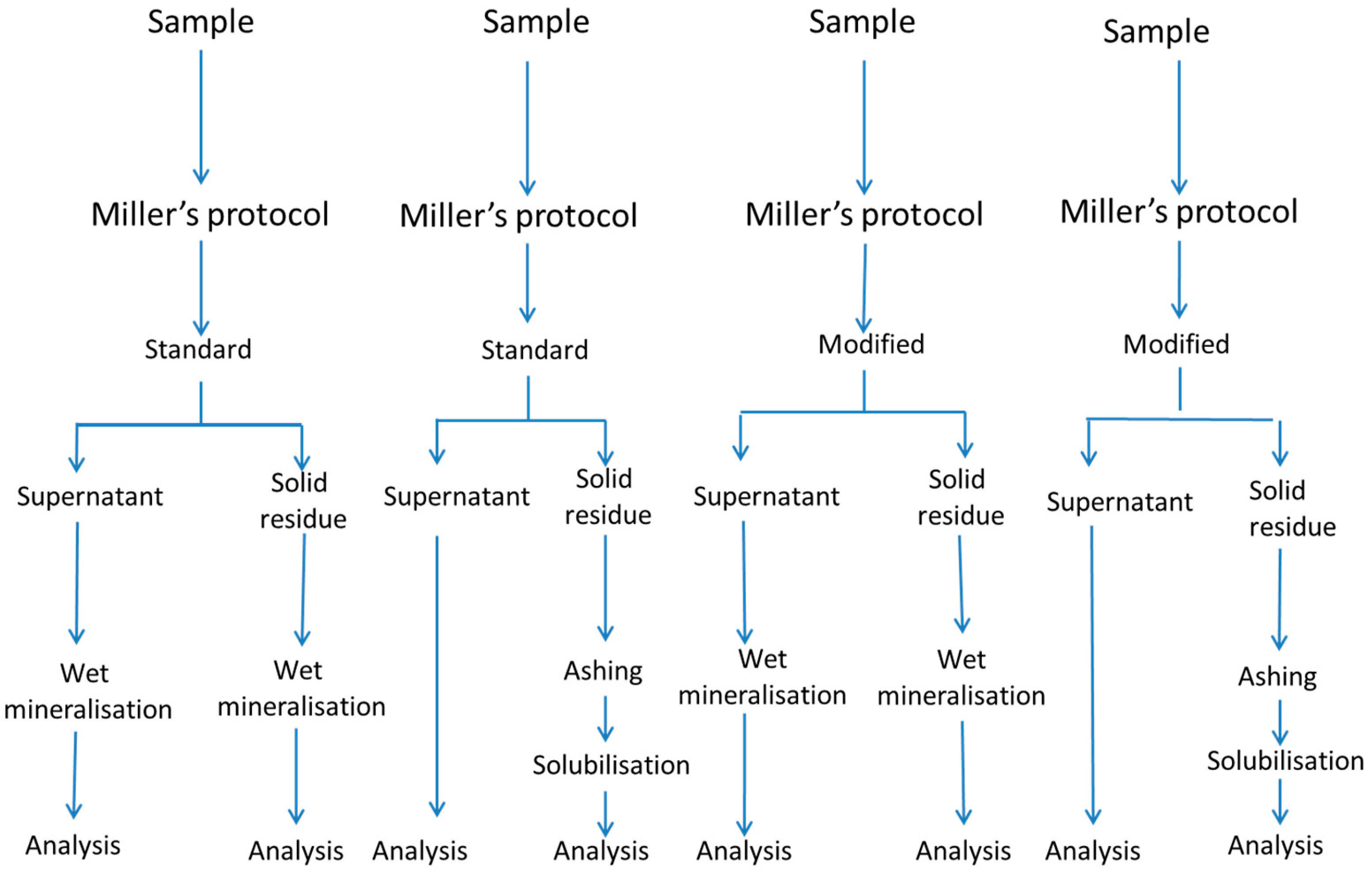
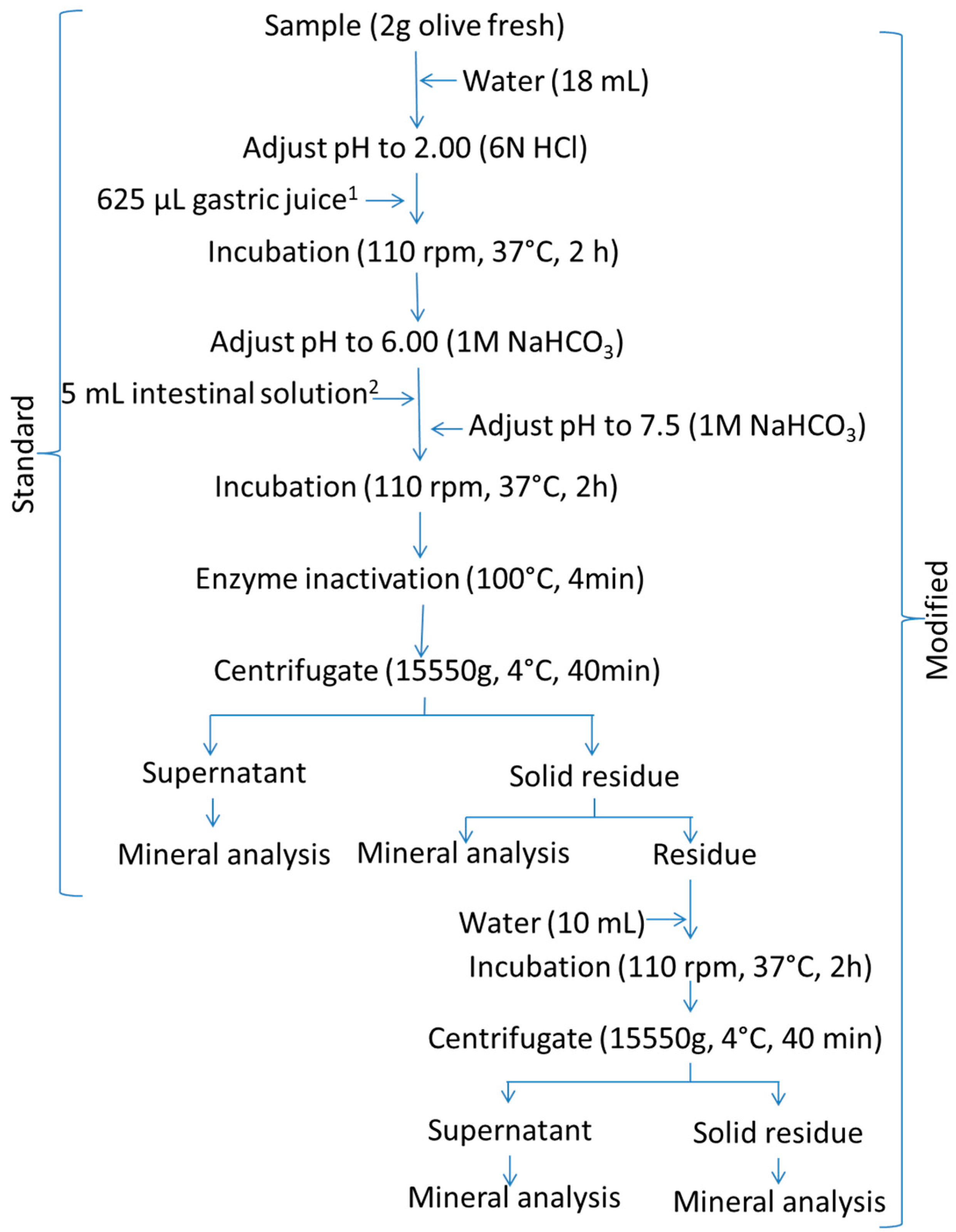
2.3.1. Miller’s Protocol
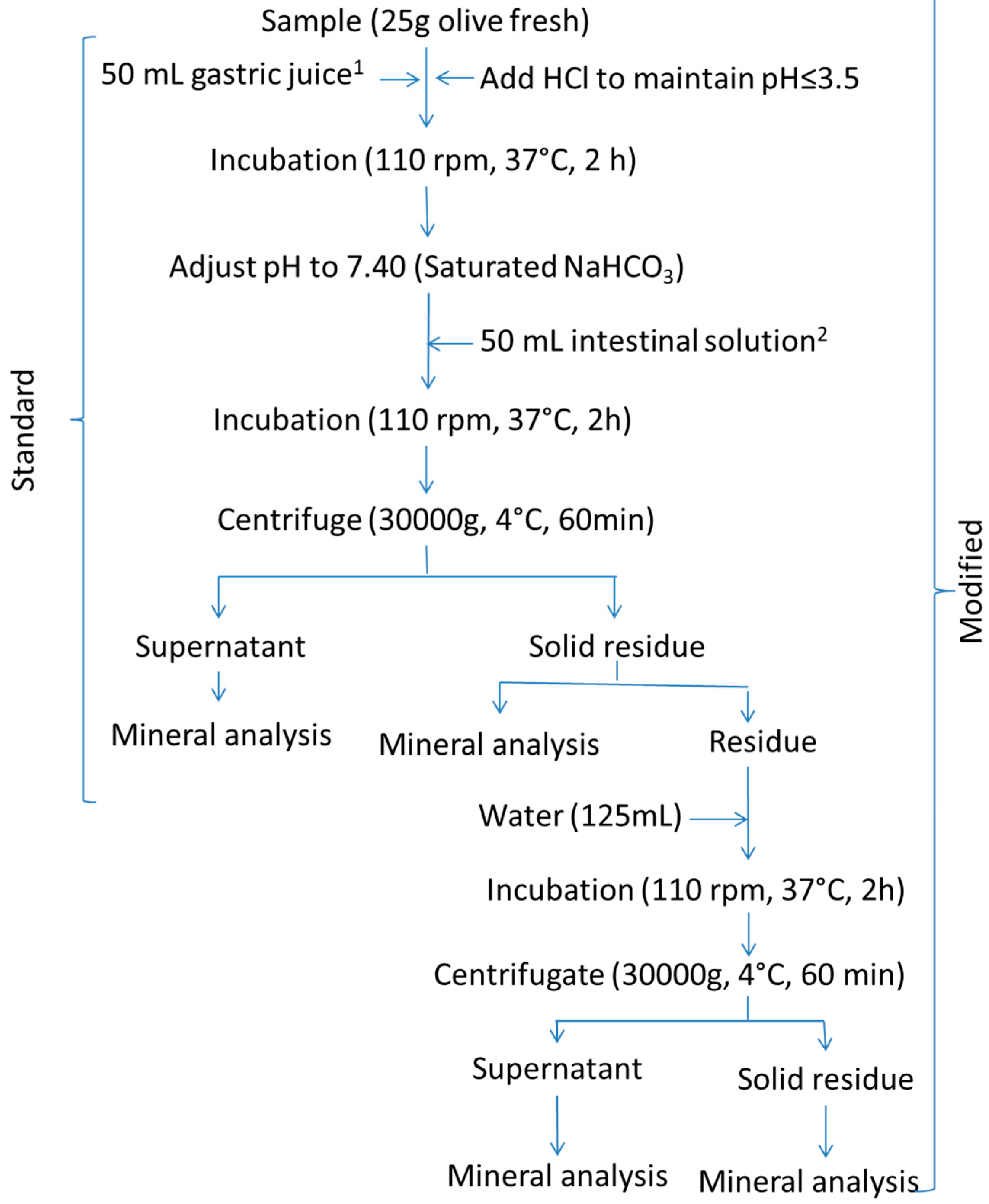
2.3.2. Crews’ Protocol
2.3.3. Modified Protocols
2.4. Mineralisation
2.4.1. Wet Mineralisation
2.4.2. Ashing
2.5. Mineral Analysis
2.6. Apparatus and Reagents
2.7. Mineral Recovery and Bioaccessibility Estimation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Mineral Linkage to Olive Flesh Components and Post-Digestion Extraction
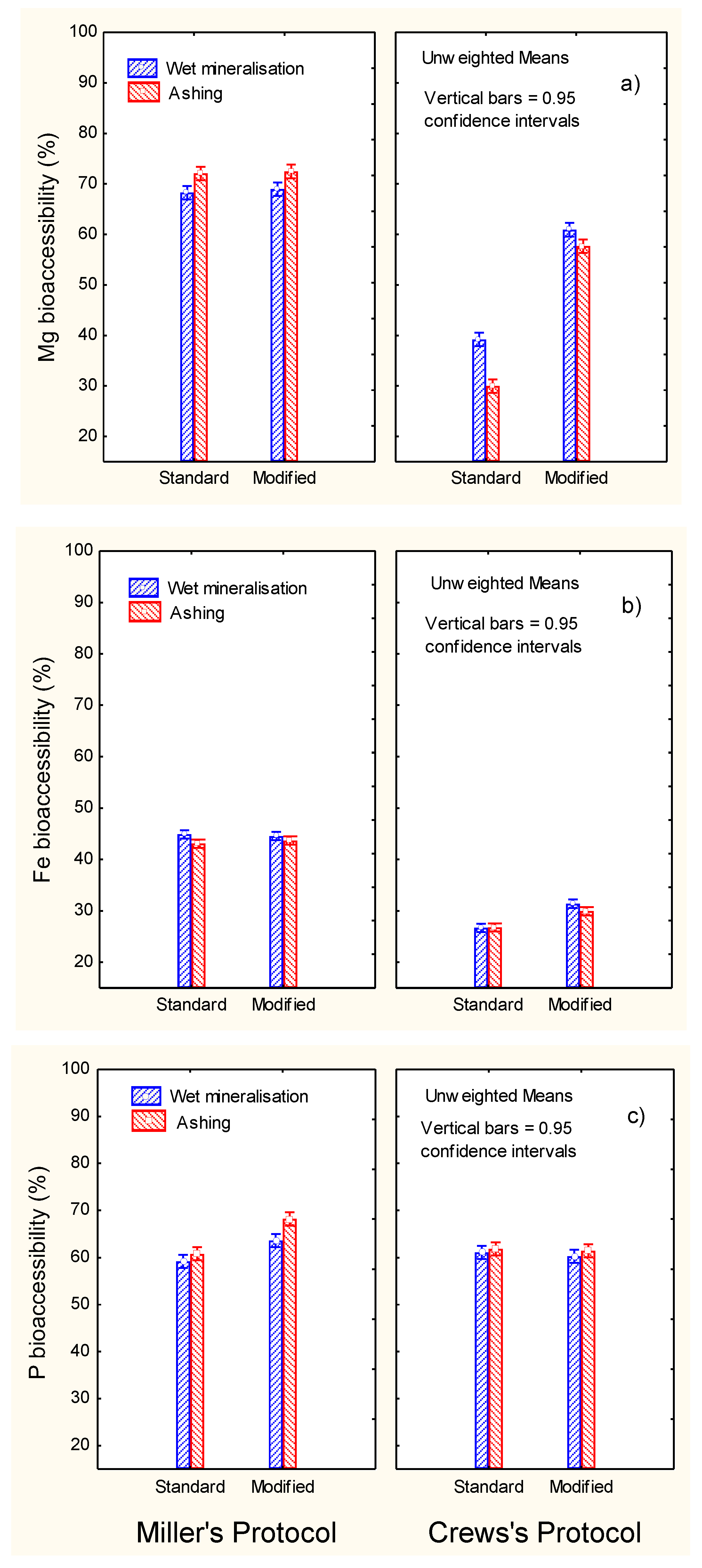
3.2. Effect of Diverse Factors on the Mineral Bioaccessibility
3.3. Mineral Recovery during Digestion
3.4. Contribution of Ripe Olives to Daily Recommended Mineral Intake
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Drug Administration, Department of Health and Human Services. Part 101.9, Nutrition Labeling of Food. In Title 21, Foods and Drugs. Electronic Code of Federal Regulations; US. Government Printing Office: Washington DC, USA, 2019. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=c9d1fccd36e3cef779a7ecf6019b2e04&mc=true&node=se21.2.101_19&rgn=div8 (accessed on 12 December 2019).
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 54, 18–61. [Google Scholar]
- The National Academies of Sciences, Engineering and Medicine, Health and Medicine Division. Dietary Reference Intakes Tables and Application. 2019. Available online: https://http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx (accessed on 19 December 2019).
- International Olive Council (IOC). World Table Olives Figures: Production. 2020. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/ (accessed on 16 February 2020).
- Garrido-Fernández, A.; Fernández-Díez, M.J.; Adams, R.M. Table Olive Production and Processing; Chapman & Hall: London, UK, 1997. [Google Scholar]
- López, A.; García, P.; Garrido, A. Multivariate characterization of table olives according to their mineral nutrient composition. Food Chem. 2008, 106, 369–378. [Google Scholar] [CrossRef]
- Miller, D.D.; Schicker, B.R.; Rasmussen, R.R.; Campen, D.V. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesias, M.; Seiquer, I.; Navarro, P. Influence of diets rich in Maillard reaction products on calcium bioavailability. Assays in male adolescents and in Caco-2 cells. J. Agric. Food Chem. 2009, 57, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Trace element solubility from food following enzymolysis. Z. Lebensm. UntersForsch 1985, 180, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Comparison of trace element solubility from food items treated separately and in combination. Z. Lebensm. UntersForsch 1985, 180, 405–410. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Montaño, A.; García, P.; Garrido, A. Fatty acid profile of table olives and its multivariate characterization using unsupervised (PCA) and supervised (DA) chemometrics. J. Agric. Food Chem. 2006, 54, 6747–6753. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Preliminary enzymolysis studies on trace element extractability from food. J. Sci. Food Agric. 1983, 34, 997–1004. [Google Scholar] [CrossRef]
- Athanasopoulos, N. GBC 932/933 Atomic Absorption Spectrophotometers; Operation Manual: Braeside, VIC, Australia, 1994. [Google Scholar]
- AOAC International. Phosphorus in fruits and fruit products no. 970.39. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 1993. [Google Scholar]
- StatSoft. Statistica for Windows (Computer Program Manual); StatSoft: Tulsa, Oklahoma, 2007. [Google Scholar]
- Miller, D.D. Minerals. In Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker: New York, NY, USA, 1996; Chapter 9; pp. 617–649. [Google Scholar]
- Jiménez, A.; Heredia, A.; Guillén, R.; Fernández-Bolaños, J. Correlation between soaking conditions, cation content of cell wall, and olive firmness during “Spanish green olive” processing. J. Agric. Food Chem. 1997, 45, 1653–1658. [Google Scholar] [CrossRef]
- Cámara, F.; Amaro, M.A.; Barberá, R.; Clemente, G. Bioaccessibility of minerals in school meals: Comparison between dialysis and solubility methods. Food Chem. 2005, 92, 481–489. [Google Scholar] [CrossRef]
- Seiquer, I.; Delgado Andrade, C.; Haro, A.; Navarro, M.P. Assessing the effects of severe heat treatment of milk on calcium bioavailability; in vitro and in vivo studies. J. Dairy Sci. 2010, 93, 5635–5643. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, D.; Heimbeck, I.; Herpich, C.; Naue, N.; Höfler, J.; Timmer, W.; Michalke, B. Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr. 2017, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, M.; Arnaud, M.J.; Kastenmayer, P.; Rytz, T.Z.; Barclay, D.V. Meal effect on mineral bioavailability from mineral water in healthy women. Am. J. Clin. Nutr. 2002, 75, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenider, I.; Greupner, T.; Hahn, A. Magnesium bioavailability from mineral waters with different mineralization levels in comparison to bread and supplement. Food Nutr. Res. 2017, 61, 1384686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karp, H.; Ekholm, P.; Kemi, V.; Hirvonen, T.; Lamberg-Allardt, C. Differences among total and in vitro digestible phosphorus content in meat and milk products. J. Ren. Nutr. 2012, 22, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Karp, H.; Ekholm, P.; Kemi, V.; Itkonen, S.; Hirvonen, T.; Nárkki, S.; Lamberg-Allardt, C. Differences among total and in vitro digestible phosphorus content in plant foods and beverages. J. Ren. Nutr. 2012, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grasses, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]

| Standard | Modified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Mineralisation | Raw Material | Supernatant Solution | Solid Olive Residue | Blank | Raw Material | Supernatant Solution | Solid Olive Residue | Blank |
| Na | Wet | 7132 (20) | 1861 (8) | 2089 (110) | 1309 (8) | 7104 (30) | 1184 (1) | 292 (14) | 1002 (5) |
| Ashing | 7181 (5) | 1752 (5) | 2738 (89) | 1215 (6) | 7085 (11) | 1257 (11) | 272 (9) | 1111 (6) | |
| K | Wet | 109.4 (0.7) | 28.0 (0.1) | 41.0 (1.7) | 20.3 (0.4) | 104.8 (1.0) | 20.2 (<0.1) | 5.2 (0.4) | 18.4 (<0.1) |
| Ashing | 107.4 (1.0) | 26.9 (<0.1) | 55.1 (3.1) | 19.4 (0.2) | 107.0 (1.5) | 21.2 (<0.1) | 4.8 (0.1) | 19.6 (<0.1) | |
| Ca | Wet | 1689.0 (8.0) | 29.7 (0.6) | 1275.7 (66.4) | 1.7 (0.0) | 1666.2 (3.6) | 20.7 (0.1) | 1449.9 (63.8) | 1.4 (<0.1) |
| Ashing | 1700.6 (6.0) | 29.5 (0.2) | 1751.5 (104.4) | 1.9 (<0.1) | 1698.4 (6.0) | 21.0 (0.1) | 1713.4 (4.1) | 1.7 (<0.1) | |
| Mg | Wet | 133.4 (0.4) | 12.4 (<0.1) | 38.3 (2.0) | 3.9 (<0.1) | 138.4 (0.2) | 9.01 (<0.1) | 45.0 (1.6) | 3.9 (<0.1) |
| Ashing | 129.7 (0.9) | 12.1 (<0.1) | 48.9 (2.7) | 3.8 (<0.1) | 128.7 (0.8) | 8.4 (<0.1) | 50.1 (0.8) | 3.7 (<0.1) | |
| Fe | Wet | 102.6 (0.8) | 4.8 (<0.1) | 55.7 (2.5) | 0.9 (<0.1) | 101.8 (0.9) | 3.0 (<0.1) | 66 (3.7) | 0.4 (<0.1) |
| Ashing | 104.5 (1.1) | 4.5 (<0.1) | 77.6 (5.4) | 0.6 (<0.1) | 104.6 (2.4) | 3.4 (<0.1) | 77.0 (0.6) | 0.7 (<0.1) | |
| P | Wet | 94.3 (0.4) | 33.2 (0.1) | 34.7 (1.0) | 25.9 (<0.1) | 91.3 (1.4) | 25.1 (<0.1) | 38.2 (1.3) | 28.1 (<0.1) |
| Ashing | 94.8 (0.6) | NA | 48.6 (2.7) | NA | 99.8 (0.8) | NA | 48.4 (0.5) | NA | |
| Standard | Modified | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Mineralisation | Raw Material | Supernatant Solution | Solid Olive Residue | Blank | Raw Material | Supernatant Solution | Solid Olive Residue | Blank |
| Na | Wet | 7120 (7) | 6139 (63) | 7940 (220) | 6139 (63) | 7121 (25) | 3653 (11) | 981 (20) | 5008 (11) |
| Ashing | 7116 (5) | 6076 (85) | 7148 (27) | 5100 (37) | 7157 (12) | 3539 (21) | 950 (30) | 5098 (13) | |
| K | Wet | 104.0 (0.8) | 44.7 (0.4) | 112.1 (2.0) | 33.4 (0.2) | 107.6 (0.3) | 28.6 (0.1) | 19.0 (1.2) | 31.3 (0.1) |
| Ashing | 105.5 (1.6) | 42.8 (0.6) | 103.2 (2.0) | 32.1 (<0.1) | 105.0 (0.7) | 27.2 (0.1) | 16.2 (0.2) | 31.3 (0.2) | |
| Ca | Wet | 1710.5 (8.3) | 1.3 (<0.1) | 2949.3 (56.8) | 0.6 (<0.1) | 1693.9 (4.6) | 3.4 (<0.1) | 2686.0 (4.6) | 0.6 (<0.1) |
| Ashing | 1702.6 (7.3) | 1.2 (<0.1) | 2585.0 (26.7) | 0.6 (<0.1) | 1709.9 (8.3) | 3.3 (<0.1) | 2580.7 (120.1) | 0.5 (<0.01) | |
| Mg | Wet | 132.0 (2.1) | 15.0 (<0.1) | 163.3 (5.3) | 6.1 (<0.1) | 132.6 (1.0) | 11.6 (<0.1) | 82.4 (3.3) | 5.3 (<0.1) |
| Ashing | 134.5 (0.7) | 14.3 (0.2) | 143.0 (1.0) | 5.6 (<0.1) | 133.5 (1.5) | 11.8 (<0.1) | 79.3 (2.9) | 6.1 (<0.1) | |
| Fe | Wet | 103.3 (2.1) | 6.0 (<0.1) | 131.0 (4.5) | 0.35 (<0.1) | 103.5 (0.7) | 3.7 (<0.1) | 125.1 (11.9) | 0.7 (<0.1) |
| Ashing | 104.6 (1.6) | 6.3 (0.1) | 119.6 (2.3) | 0.8 (<0.1) | 103.7 (1.0) | 3.7 (<0.1) | 114.1 (4.7) | 0.6 (<0.1) | |
| P | Wet | 96.2 (0.8) | 53.6 (0.5) | 69.5 (0.7) | 37.7 (0.2) | 96.1 (0.4) | 29.1 (0.1) | 59.9 (0.9) | 38.8 (0.1) |
| Ashing | 94.7 (1.1) | NA | 57.2 (0.7) | NA | 95.4 (1.6) | NA | 59.0 (1.9) | NA | |
| Technique | Type of Digestion | Mineralisation | Sample Weight | Supernatant Solution | Solid Residue | Blank Solution |
|---|---|---|---|---|---|---|
| Miller | Standard | Wet | 2.011 (0.004) | 24.020 (0.096) | 2.163 (0.094) | 26.406 (−) |
| Ashing | 2.030 (0.012) | 24.070 (0.086) | 1.599 (0.121) | 26.345 (−) | ||
| Modified | Wet | 2.022 (0.002) | 34.005 (0.088) | 1.849 (0.086) | 26.454 (−) | |
| Ashing | 2.041 (0.009) | 34.218 (0.109) | 1.622 (0.021) | 26.372 (−) | ||
| Crews | Standard | Wet | 25.060 (0.025) | 123.160 (1.194) | 14.533 (0.405) | 136.530 (−) |
| Ashing | 25.017 (0.038) | 125.802 (1.931) | 16.272 (0.265) | 138.090 (−) | ||
| Modified | Wet | 25.170 (0.067) | 236.286 (0.850) | 15.753 (0.469) | 139.150 (−) | |
| Ashing | 25.407 (0.194) | 244.145 (1.659) | 16.424 (0.594) | 136.510 (−) |
| Technique | Type of Digestion | Mineralisation | Na | K | Ca | Mg | Fe | P |
|---|---|---|---|---|---|---|---|---|
| Miller | Standard | Wet | 102.20 (0.26) | 101.32 (0.36) | 100.61 (0.48) | 103.30 (0.34) | 102.45 (0.28) | 99.45 (0.49) |
| Ashing | 99.84 (0.52) | 102.43 (0.33) | 99.90 (0.42) | 102.32 (0.24) | 102.17 (0.24) | 100.00 (NA) * | ||
| Modified | Wet | 99.59 (0.58) | 99.65 (0.28) | 99.02 (0.38) | 102.11 (0.23) | 104.03 (0.24) | 98.33 (0.42) | |
| Ashing | 97.90 (0.31) | 99.70 (0.52) | 99.86 (0.55) | 103.41 (0.17) | 103.74 (0.14) | 100.00 (NA) * | ||
| Crews | Standard | Wet | 97.98 (0.35) | 98.95 (0.62) | 100.06 (0.49) | 101.99 (0.48) | 100.16 (0.56) | 102.17 (0.40) |
| Ashing | 98.95 (0.74) | 99.95 (0.68) | 98.91 (0.59) | 99.54 (0.43) | 101.84 (0.79) | 100.00 (NA) * | ||
| Modified | Wet | 101.30 (0.49) | 99.02 (0.26) | 100.65 (0.35) | 98.27 (0.28) | 100.03 (0.54) | 99.80 (0.50) | |
| Ashing | 100.94 (0.49) | 99.08 (0.38) | 98.93 (0.42) | 98.91 (0.38) | 101.87 (0.28) | 100.00 (NA) * |
| Technique | Type of Digestion | Mineralisation | Na | K | Ca | Mg | Fe | P |
|---|---|---|---|---|---|---|---|---|
| Miller | Standard | Wet | 21.04 (0.08) | 0.336 (0.001) | 4.16 (0.06) | 1.09 (0.01) | 32.40 (0.14) | 0.808 (0.003) |
| Ashing | 20.91 (0.07) | 0.334 (<0.001) | 4.08 (0.02) | 2.52 (<0.01) | 32.88 (0.07) | 0.811 (0.005) * | ||
| Modified | Wet | 28.37 (0.15) | 0.499 (0.001) | 4.12 (0.02) | 2.68 (<0.01) | 32.76 (0.07) | 0.785 (0.004) | |
| Ashing | 28.00 (0.08) | 0.512 (0.002) | 4.12 (0.02) | 2.48 (<0.01) | 33.58 (0.08) | 0.871 (0.002) * | ||
| Crews | Standard | Wet | 9.91 (0.10) | 0.190 (<0.001) | 0.04 (<0.01) | 1.07 (<0.01) | 19.74 (0.06) | 0.828 (0.002) |
| Ashing | 9.96 (0.14) | 0.192 (<0.001) | 0.04 (<0.01) | 1.09 (<0.01) | 19.62 (0.05) | 0.822 (0.004) * | ||
| Modified | Wet | 27.52 (0.13) | 0.477 (0.001) | 0.36 (<0.01) | 2.12 (<0.01) | 22.46 (0.08) | 0.835 (0.003) | |
| Ashing | 27.55 (0.13) | 0.468 (0.001) | 0.36 (<0.01) | 2.16 (0.01) | 22.98 (0.12) | 0.820 (0.006) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. In Vitro Bioaccessibility of Ripe Table Olive Mineral Nutrients. Foods 2020, 9, 275. https://doi.org/10.3390/foods9030275
López-López A, Moreno-Baquero JM, Garrido-Fernández A. In Vitro Bioaccessibility of Ripe Table Olive Mineral Nutrients. Foods. 2020; 9(3):275. https://doi.org/10.3390/foods9030275
Chicago/Turabian StyleLópez-López, Antonio, José María Moreno-Baquero, and Antonio Garrido-Fernández. 2020. "In Vitro Bioaccessibility of Ripe Table Olive Mineral Nutrients" Foods 9, no. 3: 275. https://doi.org/10.3390/foods9030275
APA StyleLópez-López, A., Moreno-Baquero, J. M., & Garrido-Fernández, A. (2020). In Vitro Bioaccessibility of Ripe Table Olive Mineral Nutrients. Foods, 9(3), 275. https://doi.org/10.3390/foods9030275