Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Production
2.2. Experimental Design
2.3. Sample Preparation
2.4. Microbiological Analysis
2.5. Physicochemical Analysis
2.6. Statistical Analysis
3. Results
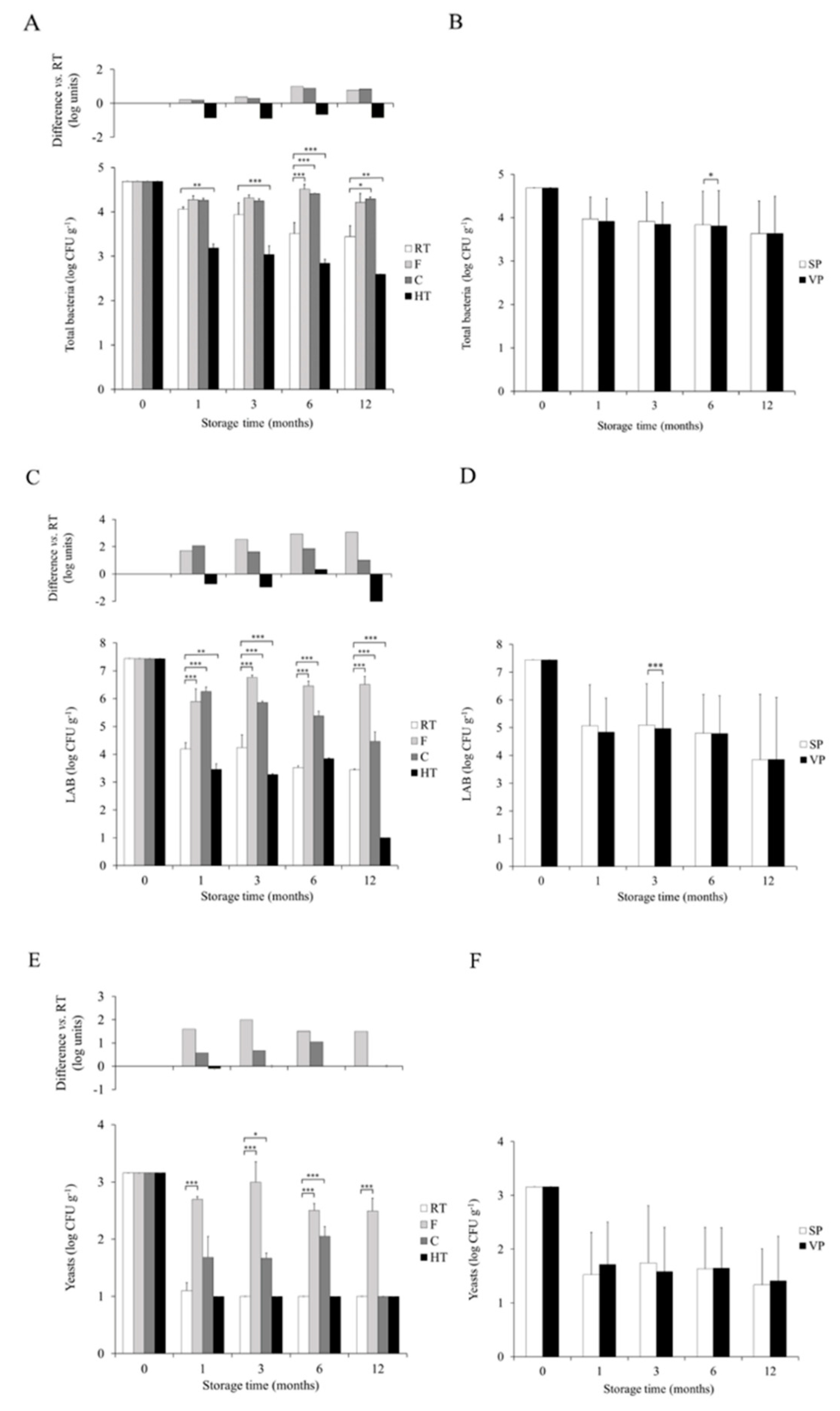
3.1. Dynamics of Total Bacteria and LAB Stored under Different Temperature and Packaging Conditions
3.2. Dynamics of Yeasts Stored under Different Temperature and Packaging Conditions
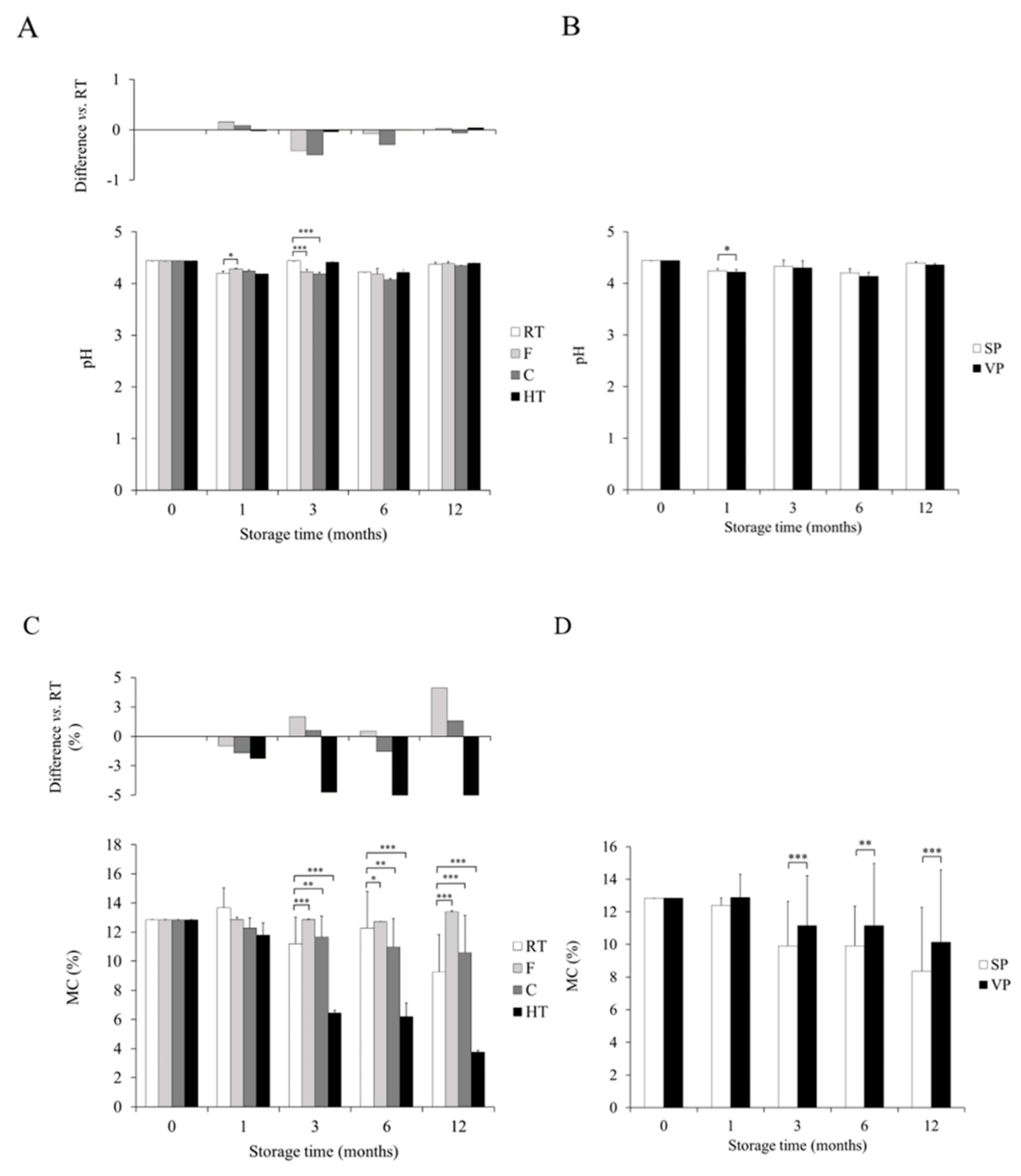
3.3. The Influence of Temperature Conditions and Packaging Modes on pH
3.4. The Influence of Temperature Conditions and Packaging Modes on Moisture Content
3.5. Interplay between Physicochemical and Microbiological Profile
4. Discussion
4.1. Bacterial Viability in FFP
4.2. Yeast Viability in FFP
4.3. Interplay between Microbial Groups
4.4. Minor Effect of Packaging Mode on FFP’s Microorganisms
4.5. pH and Moisture Content in FFP through Storage
4.6. Overall Influence of Storage on FFP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Noncommunicable Diseases Country Profiles; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Collaborators, G.B.D. 2015 R.F. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar]
- Ponder, A.; Long, M.D. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin. Epidemiol. 2013, 5, 237–247. [Google Scholar]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Fernandes, T. Nutritional Guidelines and Fermented Food Frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998, 9, 295–306. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Coda, R.; Shi, Q.; Tuomainen, P.; Katina, K.; Tenkanen, M. Exopolysaccharides Production during the Fermentation of Soybean and Fava Bean Flours by Leuconostoc mesenteroides DSM 20343. J. Agric. Food Chem. 2017, 65, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Mitchell, R. Fermentation and pathogen control: a risk assessment approach. Int. J. Food Microbiol. 2002, 79, 75–83. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, L.; Hevia, A.; Bernardo, D.; Margolles, A.; Sánchez, B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol. Lett. 2014, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A. The probiotic paradox: live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed ( Tyndallized ) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Saxelin, M.; Grenov, B.; Svensson, U.; Fondén, R.; Reniero, R.; Mattila-Sandholm, T. The technology of probiotics. Trends Food Sci. Technol. 1999, 10, 387–392. [Google Scholar] [CrossRef]
- Paul Ross, R.; Morgan, S.; Hill, C. Preservation and fermentation: past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Raja, A.; Gajalakshmi, P.; Raja, M.M.M.; Imran, M.M. Effect of Lactobacillus lactis cremoris Isolated from Kefir against Food Spoilage Bacteria. Am. J. Food Technol. 2009, 4, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, G.E.; O’Sullivan, E.; Kelly, J.; Auty, M.A.E.; Fitzgerald, G.F.; Collins, J.K.; Ross, R.P.; Stanton, C. Comparative Survival Rates of Human-Derived Probiotic Lactobacillus paracasei and L. salivarius Strains during Heat Treatment and Spray Drying. Appl. Environ. Microbiol. 2000, 66, 2605–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila-Sandholm, T.; Myllärinen, P.; Crittenden, R.; Mogensen, G.; Fondén, R.; Saarela, M. Technological challenges for future Probiotic foods. Int. Dairy J. 2002, 12, 173–182. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Hotchkiss, J.T.; Call, J.E.; Luchansky, J.B.; Phillips, J.G.; Liu, L.; Yam, K.L. Protection of probiotic bacteria in a synbiotic matrix following aerobic storage at 4 °C. Benef. Microbes 2012, 3, 175–187. [Google Scholar] [CrossRef]
- Lupien-Meilleur, J.; Roy, D.; Lagacé, L. Viability of probiotic bacteria in a maple sap beverage during refrigerated storage. LWT - Food Sci. Technol. 2016, 74, 160–167. [Google Scholar] [CrossRef]
- Shah, N.P.; Lankaputhra, W.E.V. Improving viability of Lactobacillus acidophilus and Bifidobacterium spp. in yogurt. Int. Dairy J. 1997, 7, 349–356. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Pandiella, S.S. Survival of human derived Lactobacillus plantarum in fermented cereal extracts during refrigerated storage. LWT - Food Sci. Technol. 2010, 43, 431–435. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gardner, N.J.; Roy, D. Challenges in the Addition of Probiotic Cultures to Foods. Crit. Rev. Food Sci. Nutr. 2005, 45, 61–84. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int. Dairy J. 2006, 16, 1181–1189. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Nannen, N.L. pH Homeostasis in Lactic Acid Bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Freixo, R.; Silva, J.; Gibbs, P.; Alcina, M.M.B. Paula Teixeira Dried Fruit Matrices Incorporated with a Probiotic Strain of Lactobacillus plantarum. Int. J. Food Stud. 2014, 3, 69–73. [Google Scholar] [CrossRef]
- Emser, K.; Barbosa, J.; Teixeira, P.; Morais, A.M.M.B. de Lactobacillus plantarum survival during the osmotic dehydration and storage of probiotic cut apple. J. Funct. Foods 2017, 38, 519–528. [Google Scholar] [CrossRef]
- Le Marrec, C. Responses of Lactic Acid Bacteria to Osmotic Stress. In Stress Responses of Lactic Acid Bacteria, Food Microbiology and Food Safety; Tsakalidou, E., Papadimitriou, K., Eds.; Springer: Boston, MA, USA, 2011; pp. 67–90. ISBN 9780387927718. [Google Scholar]
- Rousk, J.; Baath, E. Fungal and bacterial growth in soil with plant materials of diferent C/N ratios. FEMS Microbiol. Lett. 2007, 62, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, D.; Neffe, K.; Kolozyn-Krajewska, D.; Dolatowski, Z. Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3’/IV in dry fermented pork loins. Int. J. Food Sci. Technol. 2011, 46, 2491–2497. [Google Scholar] [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 2018, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Yu, R.C.; Chou, C.C. Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int. J. Food Microbiol. 2004, 93, 209–217. [Google Scholar] [CrossRef]
- Simpson, P.J.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J. Appl. Microbiol. 2005, 99, 493–501. [Google Scholar] [CrossRef]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Sánchez, B. Enhancing probiotic stability in industrial processes. Microb. Ecol. Heal. Dis. 2012, 23, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, S.E.; Reilly, S.S.; Kim, G.B.; Kim, H.S. Viability During Storage of Selected Probiotic Lactobacilli and Bifidobacteria in a Yogurt-like Product. J. Food Sci. 2002, 67, 3091–3095. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, L.; Süle, J.; Nagy, P. Short communication: Survival of the characteristic microbiota in probiotic fermented camel, cow, goat, and sheep milks during refrigerated storage. J. Dairy Sci. 2014, 97, 2039–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Arslan, S.; Durak, A.N.; Erbas, M.; Tanriverdi, E.; Gulcan, U. Determination of Microbiological and Chemical Properties of Probiotic Boza and Its Consumer Acceptability. J. Am. Coll. Nutr. 2015, 34, 56–64. [Google Scholar] [CrossRef]
- Rovai, M.; Salama, A.A.K. Late-Breaking Original Research; American Dairy Science Association: Knovville, TN, USA, 2018; Volume 101. [Google Scholar]
- Rovai, M.; Guifarro, L.; Anderson, J.; Salama, A.A.K. Abstracts of the 2019 American Dairy Science Association ® Annual Meeting; American Dairy Science Association: Cincinnati, OH, USA, 2019; Volume 102. [Google Scholar]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Sainz, N.; Moreno-aliaga, M.J.; Guruceaga, E. A Fermented Food Product Containing Lactic Acid Bacteria Protects ZDF Rats from the Development of Type 2 Diabetes. Nutrients 2019, 11, 2530. [Google Scholar] [CrossRef] [Green Version]
- Karimi, R.; Mortazavian, A.M.; Cruz, A.G. Da Viability of probiotic microorganisms in cheese during production and storage: a review. Dairy Sci. Technol. 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Nematollahi, A.; Sohrabvandi, S.; Mortazavian, A.M.; Jazaeri, S. Viability of probiotic bacteria and some chemical and sensory characteristics in cornelian cherry juice during cold storage. Electron. J. Biotechnol. 2016, 21, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Mortazavian, A.M.; Rehsani, M.; Mousavi, S.M.; Rezaeri, K.; Sohrabvandi, S.; Reinheimer, J. Effect of refrigerated storage temperature on the viability of probiotic micro-organisms in yogurt. Int. J. Dairy Technol. 2007, 60, 123–127. [Google Scholar] [CrossRef]
- Gharechahi, J.; Kharazian, Z.A.; Sarikhan, S.; Jouzani, G.S.; Aghdasi, M.; Salekdeh, G.H. The dynamics of the bacterial communities developed in maize silage. Microb. Biotechnol. 2017, 10, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Drywień, M.; Frąckiewicz, J.; Górnicka, M.; Gadek, J.; Jałosińska, M. Effect of prebiotic and storage time of thiamine and riboflavin content in the milk drinks fermented by Lactobacillus casei KNE-1. Rocz. Państwowego Zakładu Hig. 2015, 66, 373–377. [Google Scholar]
- Condon, S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Lett. 1987, 46, 269–280. [Google Scholar] [CrossRef]
- Dave, R.I.; Shah, N.P. Viability of Yoghurt and Probiotic Bacteria in Yoghurts Made from Commercial Starter Cultures. Int. Dairy J. 1997, 7, 31–41. [Google Scholar] [CrossRef]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Williams, J.H.; Phillips, R.D.; Chen, J. Survival of Lactobacillus rhamnosus GG as Influenced by Storage Conditions and Product Matrixes. J. Food Sci. 2012, 77, 659–663. [Google Scholar] [CrossRef]
- Daneshi, M.; Ehsani, M.R.; Razavi, S.H.; Labbafi, M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk / carrot juice mix drink. Electron. J. Biotechnol. 2013, 16, 1–12. [Google Scholar] [CrossRef]
- Iraporda, C.; Abatemarco Júnior, M.; Neumann, E.; Nunes, A.C.; Nicoli, J.R.; Abraham, A.G.; Garrote, G.L. Biological activity of the non-microbial fraction of kefir: Antagonism against intestinal pathogens. J. Dairy Res. 2017, 84, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgetti, S.R.; Vicentini, F.T.M.C.; Yokoyama, C.Y.; Borin, M.F.; Spadaro, A.C.C.; Fonseca, M.J.V. Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different β-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Rasane, P.; Jha, A.; Sharma, N. Predictive modelling for shelf life determination of nutricereal based fermented baby food. J. Food Sci. Technol. 2015, 52, 5003–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuffia, F.; Pavón, Y.; George, G.; Reinheimer, J.; Burns, P. Effect of storage temperature on the chemical, microbiological, and sensory characteristics of pasta filata soft cheese containing probiotic lactobacilli. Food Sci. Technol. Int. 2019, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tsao, M. Enhancement of survival of probiotic and non-probiotic lactic acid bacteria by yeasts in fermented milk under non-refrigerated conditions. Int. J. Food Microbiol. 2009, 135, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils - Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Talwalkar, A.; Miller, C.W.; Kailasapathy, K.; Nguyen, M.H. Effect of packaging materials and dissolved oxygen on the survival of probiotic bacteria in yoghurt. Int. J. Food Sci. Technol. 2004, 39, 605–611. [Google Scholar] [CrossRef]
- Ščetar, M.; Kovačić, E.; Kurek, M.; Galić, K. Shelf life of packaged sliced dry fermented sausage under different temperature. Meat Sci. 2013, 93, 802–809. [Google Scholar] [CrossRef]
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and a fl atoxin production by Aspergillus fl avus on paddy and polished rice. Food Chem. 2019, 293, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Zayed, G.; Roos, Y.H. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochem. 2004, 39, 1081–1086. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Shi, J.; Zhu, J.; Shao, D.; Huang, Q.; Yang, H.; Jin, M. Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl. Microbiol. Biotechnol. 2017, 101, 34–45. [Google Scholar] [CrossRef] [PubMed]

| Experimental Conditions | Sample Code | |
|---|---|---|
| Storage Temperature | Packaging Mode | |
| Freezing (−20 °C) | Standard | F-SP |
| Vacuum | F-VP | |
| Cooling (4 °C) | Standard | C-SP |
| Vacuum | C-VP | |
| Room temperature (22 °C) * | Standard | RT-SP |
| Vacuum | RT-VP | |
| High temperature (37 °C) | Standard | HT-SP |
| Vacuum | HT-VP | |
| pH | MC | Total Bacteria | LAB | Yeasts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| pH | 0.052 | 0.648 | 0.228 | 0.042 | 0.262 | 0.019 | 0.293 | 0.008 | ||
| MC | 0.052 | 0.648 | 0.557 | <0.001 | 0.618 | <0.001 | 0.616 | <0.001 | ||
| Total bacteria | 0.228 | 0.042 | 0.557 | <0.001 | 0.876 | <0.001 | 0.846 | <0.001 | ||
| LAB | 0.262 | 0.019 | 0.618 | <0.001 | 0.876 | <0.001 | 0.913 | <0.001 | ||
| Yeasts | 0.293 | 0.008 | 0.616 | <0.001 | 0.846 | <0.001 | 0.913 | <0.001 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Olmo, M.; Oneca, M.; Torre, P.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food. Foods 2020, 9, 302. https://doi.org/10.3390/foods9030302
Cabello-Olmo M, Oneca M, Torre P, Díaz JV, Encio IJ, Barajas M, Araña M. Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food. Foods. 2020; 9(3):302. https://doi.org/10.3390/foods9030302
Chicago/Turabian StyleCabello-Olmo, Miriam, María Oneca, Paloma Torre, Jesús Vicente Díaz, Ignacio J. Encio, Miguel Barajas, and Miriam Araña. 2020. "Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food" Foods 9, no. 3: 302. https://doi.org/10.3390/foods9030302






