Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Storage Conditions
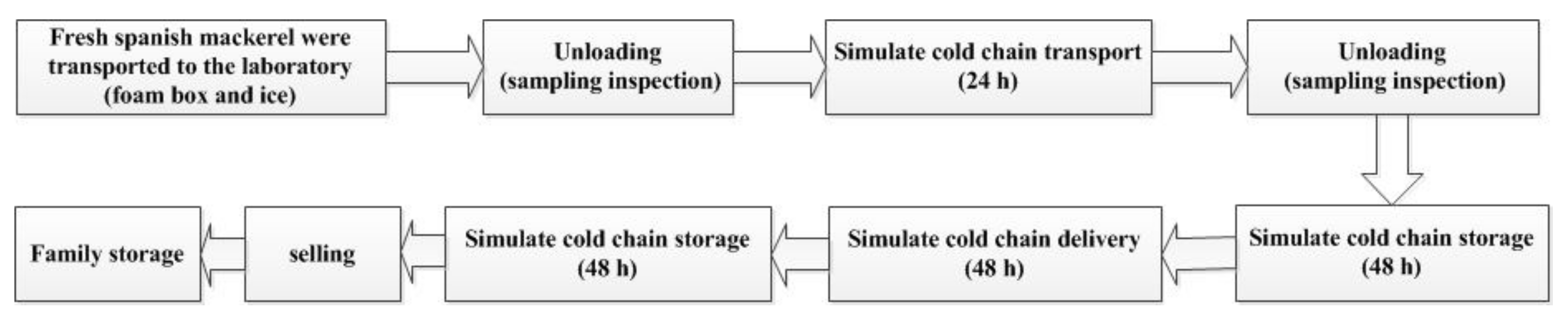
2.2. Cold-Chain Simulation Test Design
2.3. Microbiological Analysis
2.4. Chemical Analysis
2.5. Sensory Analysis
2.6. High-Throughput Sequence
2.6.1. DNA Extraction, Amplification and Sequencing
2.6.2. Data Processing and Taxonomic Classification
2.7. Statistical Analysis
3. Results and Discussion
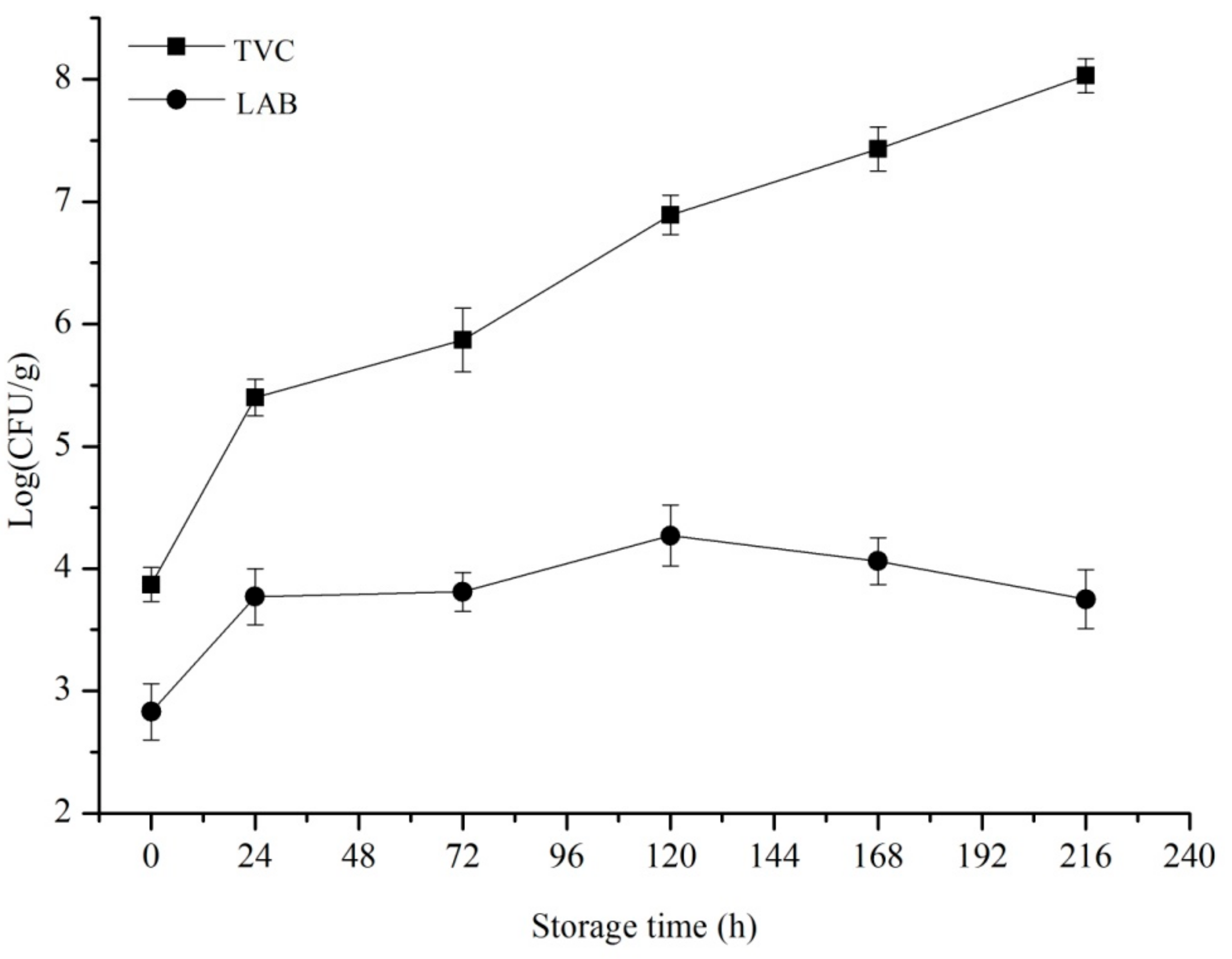
3.1. Results of Microbiological Analysis
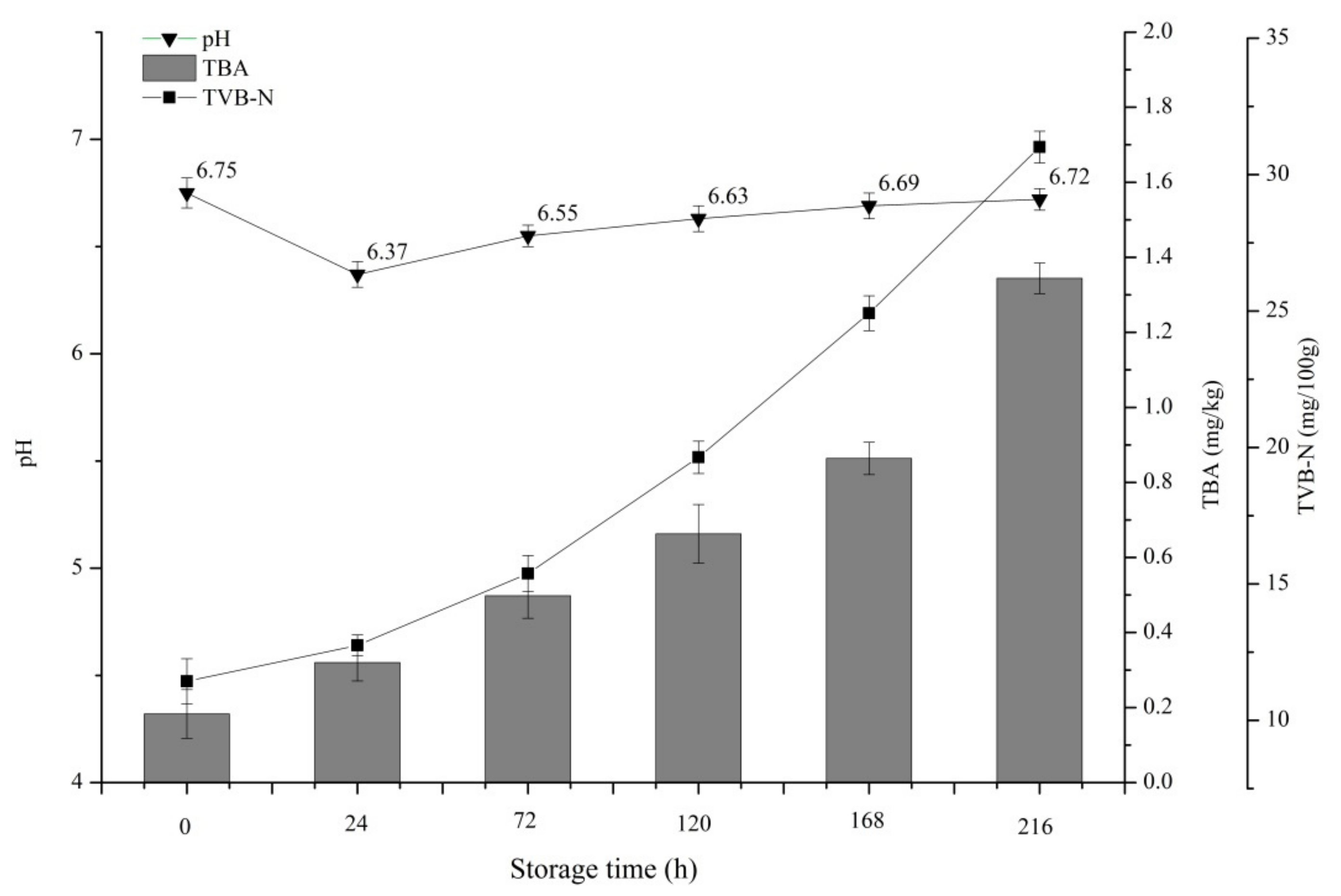
3.2. Results of Chemical Analysis
3.3. Results of Sensory Analysis
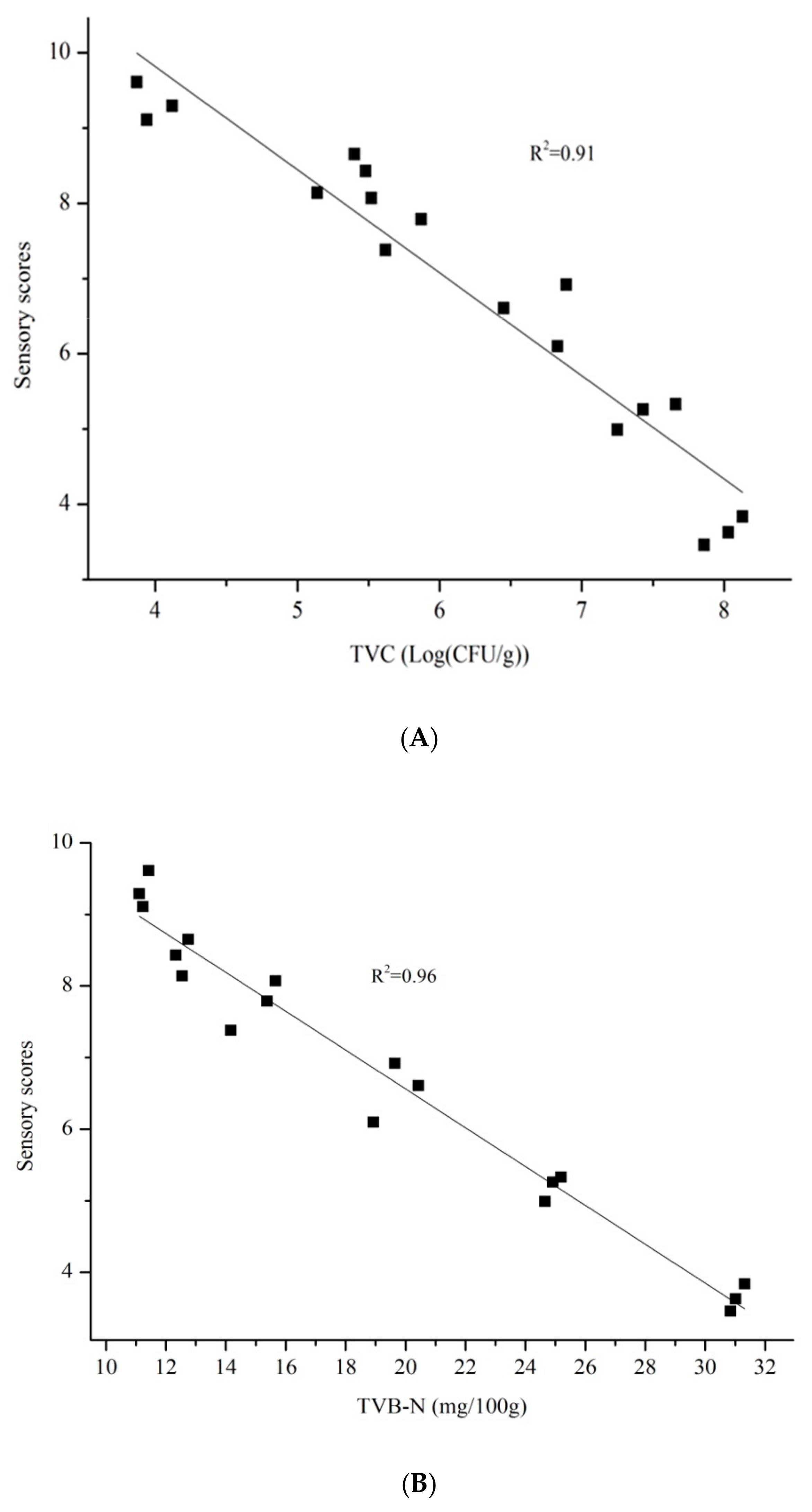
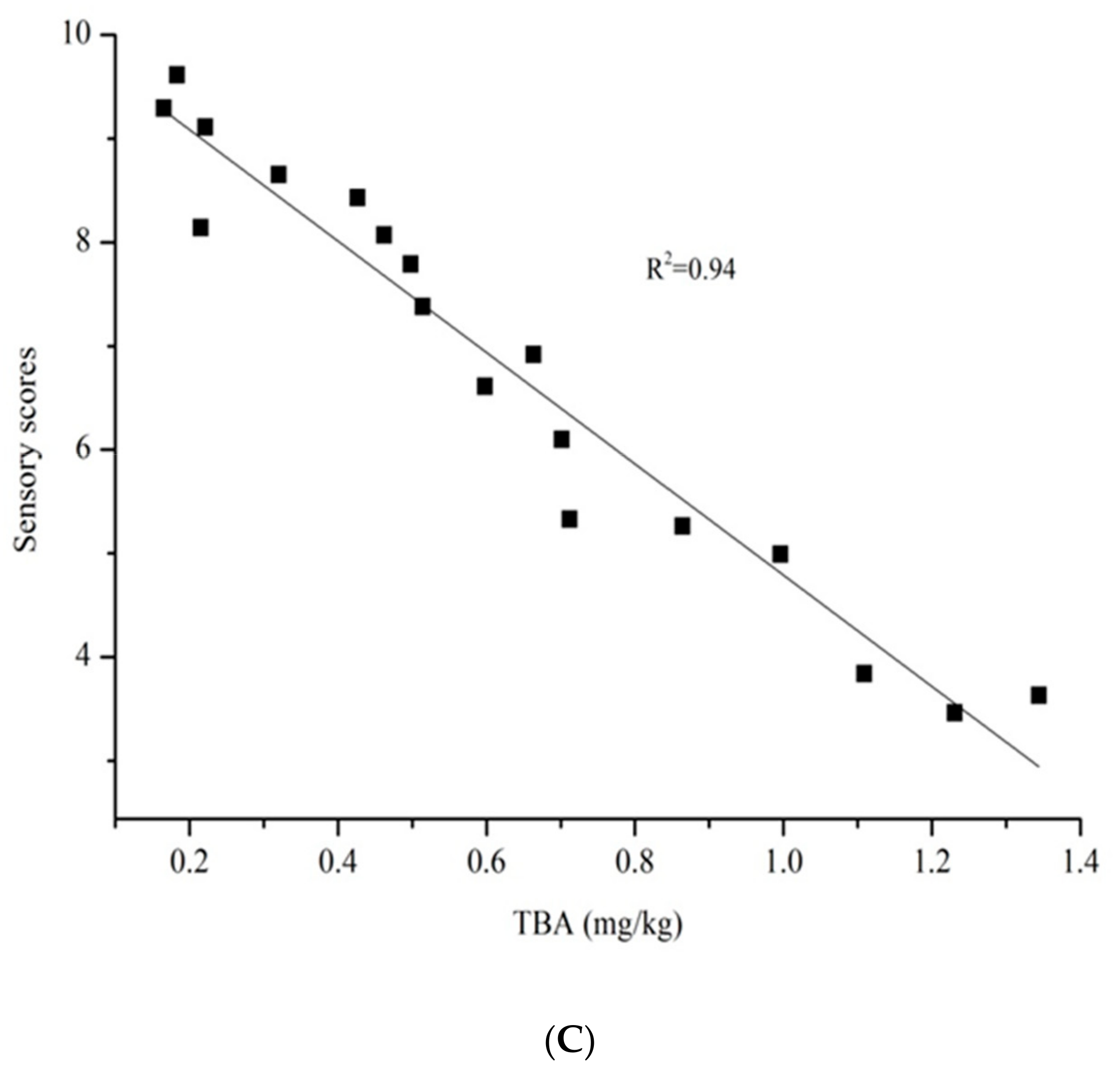
3.4. Relationship between Sensory Scores and Microbial Concentration or Chemical Indicators
3.5. Results of OTU Clustering and Species Annotation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niamaimandi, N.; Kaymaram, F.; Hoolihan, J.P.; Mohammadi, G.H.; Fatemi, S.M.R. Population dynamics parameters of narrow-barred Spanish mackerel, Scomberomorus commerson (Lacèpéde, 1800), from commercial catch in the northern Persian Gulf. Glob. Ecol. Conserv. 2015, 4, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Roa-Ureta, R.H. Stock assessment of the Spanish mackerel (Scomberomorus commerson) in Saudi waters of the Arabian Gulf with generalized depletion models under data-limited conditions. Fish. Res. 2015, 171, 68–77. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, B.; Chi, C.-F.; Zhang, Q.-H.; Gong, Y.-D.; Tang, J.-J.; Luo, H.-Y.; Ding, G.-F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, B.; Yang, G. Drying Characteristics of Spanish Mackerel during Electrohydrodynamic (EHD) Drying. In Proceedings of the 2011 Asia-Pacific Power and Energy Engineering Conference (APPEEC), Wuhan, China, 25–28 March 2011. [Google Scholar]
- Zheng, X.J.; Li, Y.P.; Wang, H.T.; Tang, B. Effect of rinsing conditions on the elastic and gel strength of surimi product. Food Sci. Technol. 2012, 37, 124–127, 133. [Google Scholar]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.A. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1B, in Spanish Mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef]
- Min, J.; Liu, H.; Li, C.Y.; Zhang, L.J.; Cao, M.J.; Liu, G.M. Preservative effects of 4 kinds of plant extracts on mackerel. J. Food Saf. Qual. 2017, 8, 2847–2855. [Google Scholar]
- Chen, T.; Lu, Y.F.; Ye, X.F.; Zhang, B. Changes of textural properties of Scomberomorus niphonius muscle from East China sea under different frozen storage conditions. Food Sci. Technol. 2012, 37, 129–132. [Google Scholar]
- Pitois, S.G.; Jansen, T.; Pinnegar, J. The impact of environmental variability on Atlantic mackerel Scomber scombrus larval abundance to the west of the British Isles. Cont. Shelf Res. 2015, 99, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Madrid, E.; Gil, F.; García, M.; Debenedetti, Á.L.; Trelis, M.; Fuentes, M.V. Potential risk analysis of human anisakiasis through the consumption of mackerel, Scomber scombrus, sold at Spanish supermarkets. Food Control 2016, 66, 300–305. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Haq, M.; Chun, B.-S. Reduction of histamine and heavy metals in mackerel hydrolyzates produced by catalysts associated-subcritical water hydrolysis. J. Ind. Eng. Chem. 2018, 68, 301–310. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Thorarinsdottir, K.A.; Olafsdottir, G. Quality Changes of Shrimp (Pandalus borealis) Stored under Different Cooling Conditions. J. Food Sci. 2010, 70, s459–s466. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria--problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 8, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Chaillou, S.; Chaulot-Talmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Hélène Desmonts, M.; Dousset, X.; Feurer, C.; Hamon, E. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2015, 9, 1105–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroi, F.; Cornet, J.; Chevalier, F.; Cardinal, M.; Coeuret, G.; Chaillou, S.; Joffraud, J.-J. Selection of bioprotective cultures for preventing cold-smoked salmon spoilage. Int. J. Food Microbiol. 2015, 213, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, C.; Ling, Z.; Huihui, S.; Qi, L. Characterization of microbial community in high-pressure treated oysters by high-throughput sequencing technology. Innov. Food Sci. Emerg. Technol. 2018, 45, 241–248. [Google Scholar] [CrossRef]
- Qi, L.; Han, Y.B.; Zhang, X.S.; Xing, S.H.; Fu, Z. Real Time Monitoring System for Aquatic Cold-chain Logistics Based on WSN. Trans. Chin. Soc. Agric. Mach. 2012, 43, 134–140. [Google Scholar]
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Carson, J.K.; East, A.R. The cold chain in New Zealand—A review. Int. J. Refrig. 2018, 87, 185–192. [Google Scholar] [CrossRef]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.-B.; Zhou, G.-H. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef]
- Yang, S.P.; Xie, J.; Gao, Z.L.; Qian, Y.F.; Shi, J. Effect of Temperature and Time Fluctuations on Quality Changes of Iced Trichiurus Haumela in Cold Chain Logistics Process. Trans. Chin. Soc. Agric. Eng. 2013, 29, 302–310. [Google Scholar]
- Ndraha, N.; Sung, W.-C.; Hsiao, H.-I. Evaluation of the cold chain management options to preserve the shelf life of frozen shrimps: A case study in the home delivery services in Taiwan. J. Food Eng. 2019, 242, 21–30. [Google Scholar] [CrossRef]
- Zhang, C.X.; Han, Y.Y.; Wang, X. Quality changes and shelf life prediction of Scomberomorus niphonius during storage. Food Ferment. Technol. 2016, 52, 101–105, 110. [Google Scholar]
- Attouchi, M.; Sadok, S. The effect of powdered thyme sprinkling on quality changes of wild and farmed gilthead sea bream fillets stored in ice. Food Chem. 2010, 119, 1527–1534. [Google Scholar] [CrossRef]
- Pacquit, A.; Frisby, J.; Diamond, D.; Lau, K.T.; Farrell, A.; Quilty, B.; Diamond, D. Development of a smart packaging for the monitoring of fish spoilage. Food Chem. 2007, 102, 466–470. [Google Scholar] [CrossRef]
- Lv, F.; Hu, Z.J.; Huang, R.J.; Ding, Y.T. Effect of Initial Central Temperature on Trachurus murphyi the Fish Quality During Refrigerated Storage. Mod. Food Sci. Technol. 2014, 30, 44–48, 68. [Google Scholar]
- Xu, Z.; Guo, Q.Y.; Yang, S. Qualitative and Quantitative Characterization of the Bacteriology of Cultured Pseudosciacna crocea in a Chilled Chain. Food Ferment. Ind. 2005, 31, 46–49. [Google Scholar]
- Macé, S.; Joffraud, J.-J.; Cardinal, M.; Malcheva, M.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon (Salmo salar) fillets stored under modified atmosphere packaging. Int. J. Food Microbiol. 2013, 160, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alvarez, O.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. The effect of several cooking treatments on subsequent chilled storage of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with different melanosis-inhibiting formulas. LWT-Food Sci. Technol. 2009, 42, 1335–1344. [Google Scholar]
- Mai, N.T.T.; Gudjónsdóttir, M.; Lauzon, H.L.; Sveinsdóttir, K.; Martinsdóttir, E.; Audorff, H.; Reichstein, W.; Haarer, D.; Bogason, S.G.; Arason, S. Continuous quality and shelf life monitoring of retail-packed fresh cod loins in comparison with conventional methods. Food Control 2011, 22, 1000–1007. [Google Scholar] [CrossRef]
- Broekaert, K.; Heyndrickx, M.; Herman, L.; Devlieghere, F.; Vlaemynck, G. Seafood quality analysis: Molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol. 2011, 28, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Pringsulaka, O.; Thongngam, N.; Suwannasai, N.; Atthakor, W.; Pothivejkul, K.; Rangsiruji, A. Partial characterisation of bacteriocins produced by lactic acid bacteria isolated from Thai fermented meat and fish products. Food Control 2012, 23, 547–551. [Google Scholar] [CrossRef]
- Dabadé, D.S.; den Besten, H.M.W.; Azokpota, P.; Nout, M.J.R.; Hounhouigan, D.J.; Zwietering, M.H. Spoilage evaluation, shelf-life prediction, and potential spoilage organisms of tropical brackish water shrimp (Penaeus notialis) at different storage temperatures. Food Microbiol. 2015, 48, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Zhang, Y.; Lu, H.; Xu, Q.; Shi, C.; Luo, Y. Spoilage potential of three different bacteria isolated from spoiled grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. LWT-Food Sci. Technol. 2017, 81, 10–17. [Google Scholar] [CrossRef]
- Don, S.; Xavier, K.A.M.; Devi, S.T.; Nayak, B.B.; Kannuchamy, N. Identification of potential spoilage bacteria in farmed shrimp (Litopenaeus vannamei): Application of Relative Rate of Spoilage models in shelf life-prediction. LWT 2018, 97, 295–301. [Google Scholar] [CrossRef]
- Bergmann, G.T.; Bates, S.T.; Eilers, K.G.; Lauber, C.L.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 2011, 43, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Hatamoto, M.; Nakahara, N.; Abe, K.; Takahashi, M.; Araki, N.; Yamaguchi, T. Community Composition of Known and Uncultured Archaeal Lineages in Anaerobic or Anoxic Wastewater Treatment Sludge. Microb. Ecol. 2015, 69, 586–596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Qiong, W.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar]
- Mehta, N.K.; Elavarasan, K.; Reddy, A.M.; Shamasundar, B.A. Effect of ice storage on the functional properties of proteins from a few species of fresh water fish (Indian major carps) with special emphasis on gel forming ability. J. Food Sci. Technol. 2014, 51, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, B.M.; Parker, T.C.; Gould, G.W. The microbiological safety and quality of food. Toxigenic Fungi Mycotoxin 2000, 57, 209–215. [Google Scholar]
- Otero, L.; Pérez-Mateos, M.; Holgado, F.; Márquez-Ruiz, G.; López-Caballero, M.E. Hyperbaric cold storage: Pressure as an effective tool for extending the shelf-life of refrigerated mackerel (Scomber scombrus, L.). Innov. Food Sci. Emerg. Technol. 2018, 51, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.R.; Raju, C.V.; Lakshmisha, I.P.; Singh, R.R. Antioxidant and antimicrobial properties of grape and papaya seed extracts and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. J. Food Sci. Technol. 2016, 53, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, L.; Pérez-Mateos, M.; López-Caballero, M.E. Hyperbaric cold storage versus conventional refrigeration for extending the shelf-life of hake loins. Innov. Food Sci. Emerg. Technol. 2017, 41, 19–25. [Google Scholar] [CrossRef]
- Kuswandi, B.; Larasati, T.S.; Abdullah, A.; Heng, L.Y. Real-Time Monitoring of Shrimp Spoilage Using On-Package Sticker Sensor Based on Natural Dye of Curcumin. Food Anal. Methods 2012, 5, 881–889. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Dogruyol, H.; Mol, S. Effect of Irradiation on Shelflife and Microbial Quality of Cold-Stored Sous-Vide Mackerel Fillets: Effect of Irradiation on Sous-Vide Fish. J. Food Process. Preserv. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Sci. Technol. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and quality changes in fresh fish. Fao Fish. Tech. Pap. 1995, 348, 195. [Google Scholar]
- Wang, S.; Xie, J.; Yang, K.; Qian, Y. The Correlation between Water Distribution and Texture, Freshness and Sensory Quality of Salmon during Low Temperature Logistics. J. Chin. Inst. Food Sci. Technol. 2018, 18, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.T. Study on the Quality Changes of Frozen Spanish Mackerel; Dalian Polytechnic University: Dalian, China, 2015. [Google Scholar]
- Pinter, N.; Maltar-Strmečki, N.; Kozačinski, L.; Njari, B.; Cvrtila Fleck, Ž. Impact of radiation treatment on chemical, biochemical and sensory properties, and microbiological quality of mackerel. Radiat. Phys. Chem. 2015, 117, 23–25. [Google Scholar] [CrossRef]
- Fan, W.; Chi, Y.; Zhang, S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Huang, W.B.; Xie, J.; Luo, C.; Wu, X.Y.; Dong, H. Effect of Temperature Fluctuation on Quality Changes of Red Drum (Sciaenops ocellatus) in Cold Chain Logistics. Food Sci. 2016, 37, 268–274. [Google Scholar]
- Bennour, M.; Marrakchi, A.E.; Bouchriti, N.; Hamama, A.; Ouadaa, M.E. Chemical and Microbiological Assessments of Mackerel (Scomber scombrus) Stored in Ice. J. Food Prot. 1991, 54, 789–792. [Google Scholar] [CrossRef]
- Izci, L.; Günlü, A.; Bilgin, S. Production of fish chips from sand smelt (Atherina boyeri, RISSO 1810) and determination of some quality changes. Iran. J. Fish. Sci. 2011, 10, 230–241. [Google Scholar]
- Mendes, R.; Silva, H.A.; Nunes, M.L.; Empis, J.M.A. Deteriorative changes during ice storage of irradiated blue Jack Mackerel (Trachurus Picturatus). J. Food Biochem. 2010, 24, 89–105. [Google Scholar] [CrossRef]
- Cheng, S.H.; Tang, H.Q.; Ou, C.R.; Zhang, M.S.; Zan, C.L.; Li, Y.M. Comparative Analysis of Compositions and Metabolic Functions of Bacterial Communities on the Surface of Mackerel and Large Yellow Croaker during Refrigerated Storage. Food Sci. 2018, 39, 218–225. [Google Scholar]
- Cao, R.; Zhang, J.; Meng, H.; Zhao, L.; Liu, Q. Microbial Flora Analysis of Oyster: A Comparison between Traditional Plate Culture Method and High Throughput Sequencing. Food Sci. 2016, 37, 137–141. [Google Scholar]
- Mohamed, F.; Kow, F.; Ngwenya, E. Relative Microbial Spoilage of Un-gutted and Gutted Cultured Edible Barramundi (Lates calcarifer). Food Stud. Interdiscip. J. 2013, 2, 31–41. [Google Scholar] [CrossRef]
- Cakli, S.; Kilinc, B.; Cadun, A.; Tolasa, S. Effects of using slurry ice on the microbiological, chemical and sensory assessments of aquacultured sea bass ( Dicentrarchus labrax ) stored at 4 °C. Crit. Rev. Food Sci. Nutr. 2006, 222, 130–138. [Google Scholar]

| Score | Whole Fish | ||||
|---|---|---|---|---|---|
| Body Surface | Odor | Fish Gills | Fish Elasticity | Eyes | |
| 9–10 | Lustrous body surface intact without mucus | Odor inherent in fish | Bright red or burgundy with transparent mucus | Muscle is firm; tissue is tight and elastic | Full eyeball and clear cornea |
| 7–8 | Lustrous, slime transparent | Natural smell, no odor | Red; mucus is more transparent | Firm and elastic; the finger depressions recover quickly after pressing | Eyeball is flat and cornea is clear |
| 5-6 | Luster is a bit poor | Natural odors, slight odors | Reddish or dark red, mucus slightly cloudy | Muscles are softer and less elastic | Eyeball is flat or slightly sunkenand cornea is slightly cloudy |
| 3–4 | Surface of the body is dim with poor luster | Stinky | Mucus is cloudy and reddish | Poor elasticity; depression recovery after compression is slow | Eyeball is sunken and bleached; cornea is cloudy |
| 1–2 | Surface of the body is dull | Distinct odor of ammonia | Soil is yellow and the mucus is cloudy | Meat is loose | Eyeball is white; cornea is seriously turbid |
| Index | Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 24 | 72 | 120 | 168 | 216 | |
| Body surface | 9.78 ± 0.17 | 8.81 ± 0.23 | 8.03 ± 0.12 | 6.98 ± 0.19 | 5.74 ± 0.24 | 4.48 ± 0.28 |
| Odor | 9.55 ± 0.14 | 8.58 ± 0.22 | 7.61 ± 0.24 | 6.82 ± 0.17 | 5.34 ± 0.45 | 3.55 ± 0.33 |
| Fish gills | 9.61 ± 0.15 | 8.52 ± 0.14 | 7.81 ± 0.27 | 6.86 ± 0.17 | 5.19 ± 0.25 | 3.51 ± 0.25 |
| Fish elasticity | 9.65 ± 0.15 | 8.70 ± 0.15 | 7.84 ± 0.14 | 6.62 ± 0.24 | 5.06 ± 0.15 | 3.40 ± 0.30 |
| Eyes | 9.45 ± 0.25 | 8.61 ± 0.17 | 7.66 ± 0.25 | 6.83 ± 0.15 | 4.96 ± 0.15 | 3.20 ± 0.13 |
| Overall assessment | 9.61 ± 0.11 | 8.65 ± 0.10 | 7.79 ± 0.015 | 6.82 ± 0.12 | 5.26 ± 0.27 | 3.63 ± 0.44 |
| Groups | Effective Tags | Bases Number/bp | Average Length/bp | OTU Number |
|---|---|---|---|---|
| M0 | 41386 | 18587195 | 449.12 | 196 |
| M24 | 46608 | 20971270 | 449.95 | 47 |
| M72 | 42977 | 19314316 | 449.41 | 36 |
| M120 | 51234 | 23051996 | 449.94 | 56 |
| M168 | 62616 | 28039637 | 447.80 | 73 |
| M216 | 55179 | 24609908 | 446.00 | 243 |
| Groups | Shannon Index | Simpson Index | ACE Index | Chao1 Index | Coverage (%) |
|---|---|---|---|---|---|
| M0 | 2.39 | 0.15 | 174.84 | 120.21 | 0.9997 |
| M24 | 2.34 | 0.18 | 123.40 | 108.00 | 0.9996 |
| M72 | 1.92 | 0.29 | 109.21 | 84.11 | 0.9981 |
| M120 | 1.95 | 0.25 | 196.67 | 137.27 | 0.9993 |
| M168 | 2.47 | 0.13 | 328.33 | 182.10 | 0.9997 |
| M216 | 2.03 | 0.22 | 241.54 | 138.15 | 0.9974 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Xu, X.; Xing, J.; Cheng, H.; Zhang, S.; Shen, J.; Li, H. Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics. Foods 2020, 9, 312. https://doi.org/10.3390/foods9030312
Zheng R, Xu X, Xing J, Cheng H, Zhang S, Shen J, Li H. Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics. Foods. 2020; 9(3):312. https://doi.org/10.3390/foods9030312
Chicago/Turabian StyleZheng, Ruihang, Xiaorong Xu, Jiali Xing, Hai Cheng, Shufen Zhang, Jian Shen, and Hesheng Li. 2020. "Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics" Foods 9, no. 3: 312. https://doi.org/10.3390/foods9030312
APA StyleZheng, R., Xu, X., Xing, J., Cheng, H., Zhang, S., Shen, J., & Li, H. (2020). Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics. Foods, 9(3), 312. https://doi.org/10.3390/foods9030312




