Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Preparation of Wood Extracts
2.3. High-Performance Thin-Layer Chromatography and Image Analysis
2.4. Principal Component Analysis
2.5. Bacterial Strains and Growth Conditions
2.6. Well-Diffusion Method
2.7. MIC Assay
3. Results and Discussion
3.1. Line Profiles of Investigated Extracts
3.2. DPPH-HPTLC Assay
3.3. Principal Component Analysis
3.4. Well-Diffusion Method
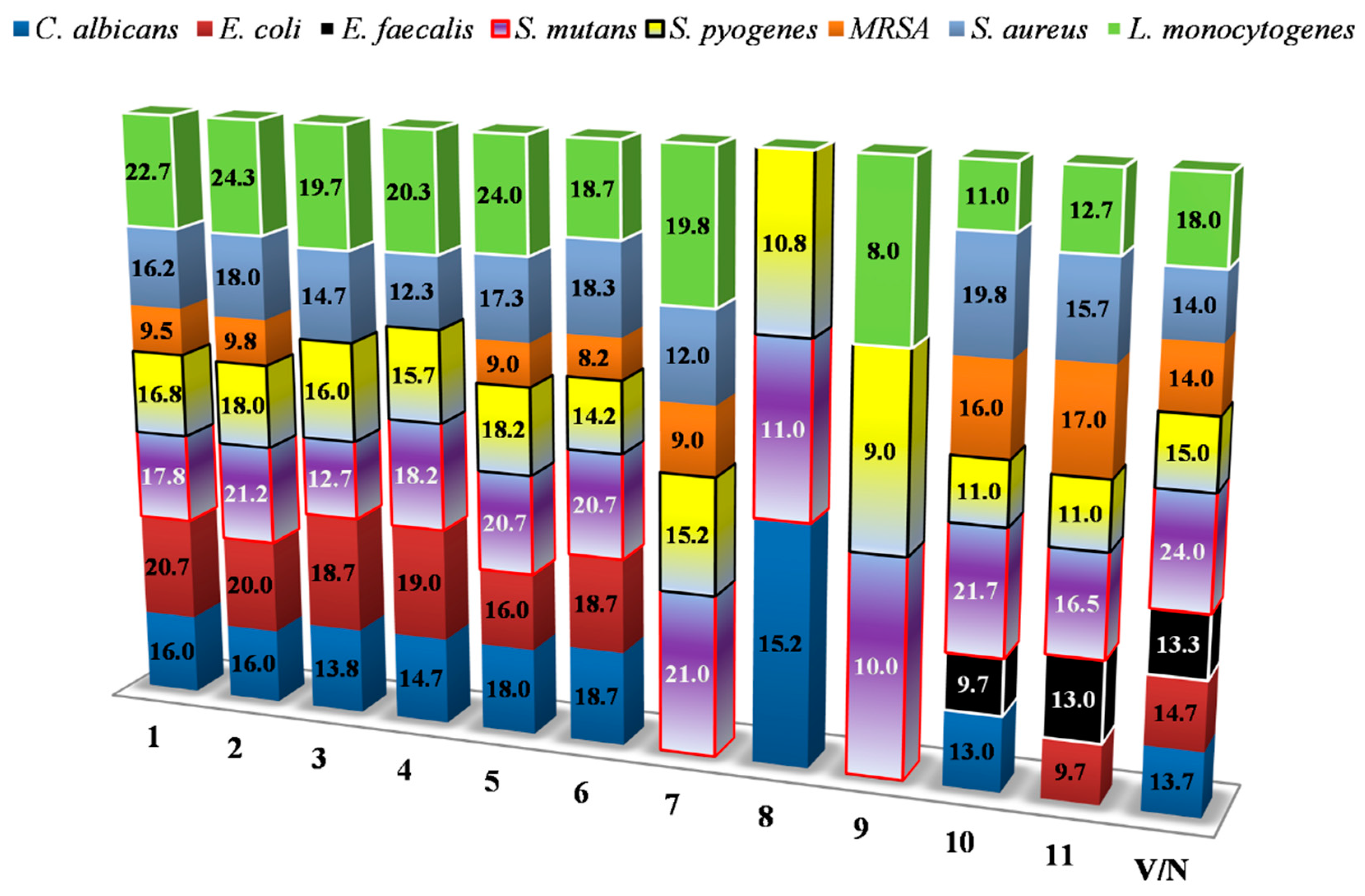
3.5. MIC Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Martínez, J.; Esteruelas, E.; Muñoz, A.M. Polyphenolic compounds as chemical markers of wine ageing in contact with cherry, chestnut, false acacia, ash and oak wood. Food Chem. 2014, 143, 66–76. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La Cara, f.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, R.M.; Matias, A.A. Polyphenol-rich extracts obtained from winemaking waste streams as natural ingredients with cosmeceutical potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Corsini, A.; Boschi, N.; Rasplolli Galletti, A.M. Smart valorization of waste biomass: Exhausted lemon peels, coffee silverskins and paper wastes for the production of levulinic acid. Chem. Eng. Trans. 2018, 65, 637–642. [Google Scholar] [CrossRef]
- Smailagić, A.; Veljović, S.; Gašić, U.; Dabić Zagorac, D.; Stanković, M.; Radotić, K.; Natić, M. Phenolic profile, chromatic parameters and fluorescence of different woods used in Balkan cooperage. Ind. Crop Prod. 2019, 132, 156–167. [Google Scholar] [CrossRef]
- Mratinić, E.; Fotirić-Akšić, M. Indigenous fruit species as a significant resource for sustainable development. Bull Fac Forest. 2014, 181–194. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Matović, B.; Orlović, S.; Kržič, A.; Trudić, B.; Galić, Z.; Stojnić, S.; Pekeč, S. Future of the Main Important Forest Tree Species in Serbia from the Climate Change Perspective. South East Eur For. 2014, 5, 117–124. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Rakonjac, V.; Mratinić, E.; Jovković, R.; Fotirić Akšić, M. Analysis of morphological variability in wild cherry (Prunus avium L.) genetic resources from Central Serbia. J. Agr. Sci. Tech. 2014, 16, 151–162. [Google Scholar]
- Mratinić, E.; Fotirić-Akšić, M.; Jovković, R. Analysis of wild sweet cherry (Prunus avium L.) germplasm diversity in South-East Serbia. Genetika 2012, 44, 259–268. [Google Scholar]
- Mihajlović, L.; Glavendekić, M.; Jakovljević, I.; Marjanović, S. Obolodiplosis robiniae (haldeman) (diptera: Cecidomyiidae) a new invasive insect pest on black locust in Serbia. Bullet. Faculty For. 2008, 97, 197–208. [Google Scholar] [CrossRef]
- Sanz, M.; Fernandez de Simón, B.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, M.T.; Estrella, I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta. 2012, 732, 33–45. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Bellachioma, A.; Versari, A.; Riponi, C. Changes in phenolic composition of red wines aged in cherry wood. LWT Food Sci. Technol. 2015, 60, 977–984. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín Gutiérrez, I.; Gordon, M.H.; Pérez –Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590. [Google Scholar] [CrossRef]
- Cieśla, Ł.; Kryszeń, J.; Stochmal, A.; Oleszek, W.; Waksmundzka-Hajnos, M. Approach to develop a standardized TLC-DPPH• test for assessing free radical scavenging properties of selected phenolic compounds. J. Pharm. Biomed. Anal. 2012, 70, 126–135. [Google Scholar] [CrossRef]
- Alañón, M.E.; García-Ruíz, A.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Moreno-Arribas, M.V. Antimicrobial and antioxidant activity of pressurized liquid extracts from oenological woods. Food Control. 2015, 50, 581–588. [Google Scholar] [CrossRef]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2010, 38, 99–107. [Google Scholar] [CrossRef]
- Asmi, K.S.; Lakshmi, T.S.; Balusamy, R.; Parameswari, R. Therapeutic aspects of taxifolin—An update. J. Adv. Pharmacy Educ. Res. 2017, 187–189. [Google Scholar]
- Joung, D.K.; Mun, S.H.; Choi, S.H.; Kang, O.H.; Kim, S.B.; Lee, Y.S.; Zhou, T.; Kong, R.; Choi, J.G.; Shin, D.W.; et al. Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism. Exp. Ther. Med. 2016, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, M.X.; Wang, T.M.; Li, Y.; Wang, C.Z. The roles of CDR1, CDR2, and MDR1 in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016, 54, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Andrenšek, S.; Simonovska, B.; Vovk, I.; Fyhrquist, P.; Vuorela, H.; Vuorela, P. Antimicrobial and antioxidative enrichment of oak (Quercus robur) bark by rotation planar extraction using ExtraChrom R. Int. J. Food Microbiol. 2004, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; El-Abedin, T.K.Z.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Bollareddy, E.; Keskar, K.; Thorat, A.; Crankshaw, D.J.; Holloway, A.C.; Khan, G.; Wright, G.D.; Ejim, L. Isolation of flavonoids from the heartwood and resin of Prunus avium and some preliminary biological investigations. Phytochemistry 2009, 70, 2040–2046. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Ristivojević, P.; Andrić, F.L.; Trifković, J.Đ.; Vovk, I.; Stanisavljević, L.Ž.; Tešić, Ž.L.; Milojković-Opsenica, D.M. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J. Chemom. 2014, 28, 302–310. [Google Scholar] [CrossRef]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crop Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crop Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Let. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Kosalec, I. Galangin expresses bactericidal activity against multiple-resistant bacteria: MRSA, Enterococcus spp. and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2004, 240, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; Elansary, H.O.; Elkelish, A.A.; Zeidler, A.; Ali, H.M.; Mervat, E.H.; Yessoufou, K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. Bioresources. 2016, 11, 9421–9437. [Google Scholar] [CrossRef]
- Brantner, A.; Grein, E. Antibacterial activity of plant extracts used externally in traditional medicine. J. Ethnopharmacol. 1994, 44, 35–40. [Google Scholar] [CrossRef]
- Berahou, A.; Auhmani, A.; Fdil, N.; Benharref, A.; Jana, M.; Gadhi, C.A. Antibacterial activity of Quercus ilex bark’s extracts. J. Ethnopharmacol. 2007, 112, 426–429. [Google Scholar] [CrossRef]
- Khouzami, L.; Mroueh, M.; Daher, C.F. The role of methanolic extract of Quercus infectoria bark in lipemia, glycemia, gastric ulcer and bacterial growth. J. Med. Plants Res. 2009, 2, 224–230. [Google Scholar]
- Lamounier, K.C.; Cunha, L.C.S.; de Morais, S.A.L.; de Aquino, F.J.T.; Chang, R.; do Nascimento, E.A.; de Souza, M.G.M.; Martins, C.H.G.; Cunha, W.R. Chemical analysis and study of phenolics, antioxidant activity, and antibacterial effect of the wood and bark of Maclura tinctoria (L.) D. Don ex Steud. Evid. Based Complement. Alternat. Med. 2012, 451039. [Google Scholar] [CrossRef][Green Version]
- Bii, C.; Korir, K.R.; Rugutt, J.; Mutai, C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. J. Med. Plants Res. 2010, 4, 995–998. [Google Scholar] [CrossRef]
- Oyetayo, A.M.; Bada, S.O. Phytochemical screening and antibacterial activity of Prunus avium extracts against selected human pathogens. J. Complement. Altern. Med. Res. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Arora, D.S.; Mahajan, H. In vitro evaluation and statistical optimization of antimicrobial activity of Prunus cerasoides stem bark. Appl. Biochem. Biotechnol. 2018, 184, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Fozing, D.C.; Kapche, W.F.G.D.; Mbaveng, A.T.; Kuiate, J.R.; Ngadjui, B.T.; Abegaz, B.M. Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J. Ethnopharmacol. 2009, 124, 551–555. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.G.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial Activity of Tunisian Quince (Cydonia oblonga Miller) Pulp and Peel Polyphenolic Extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
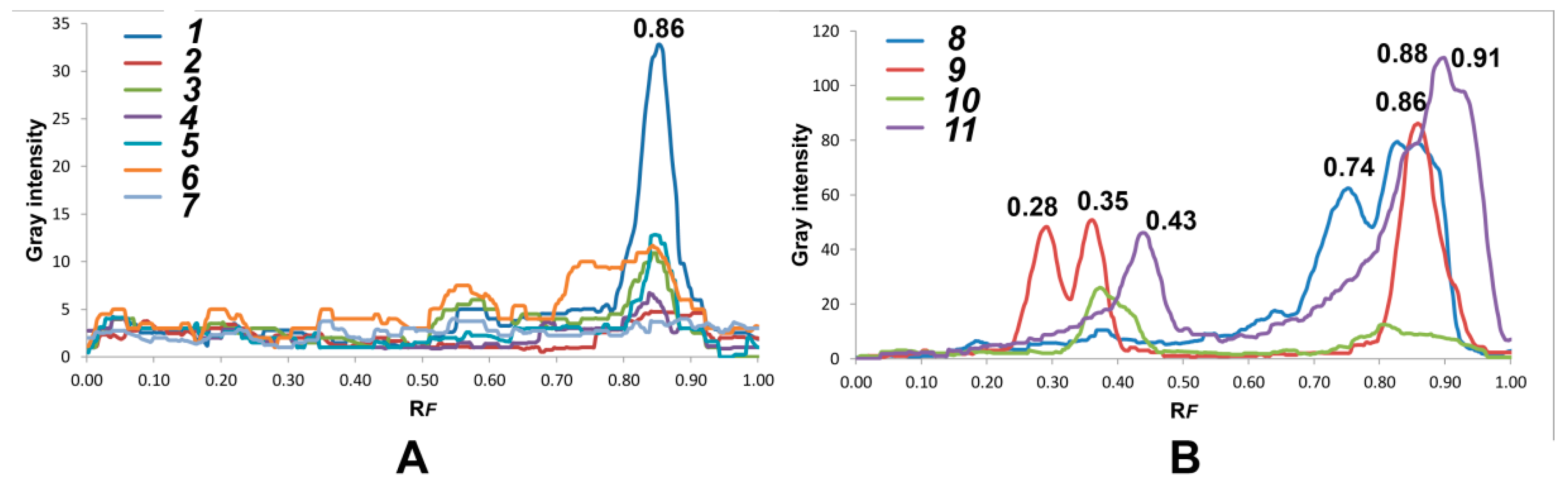
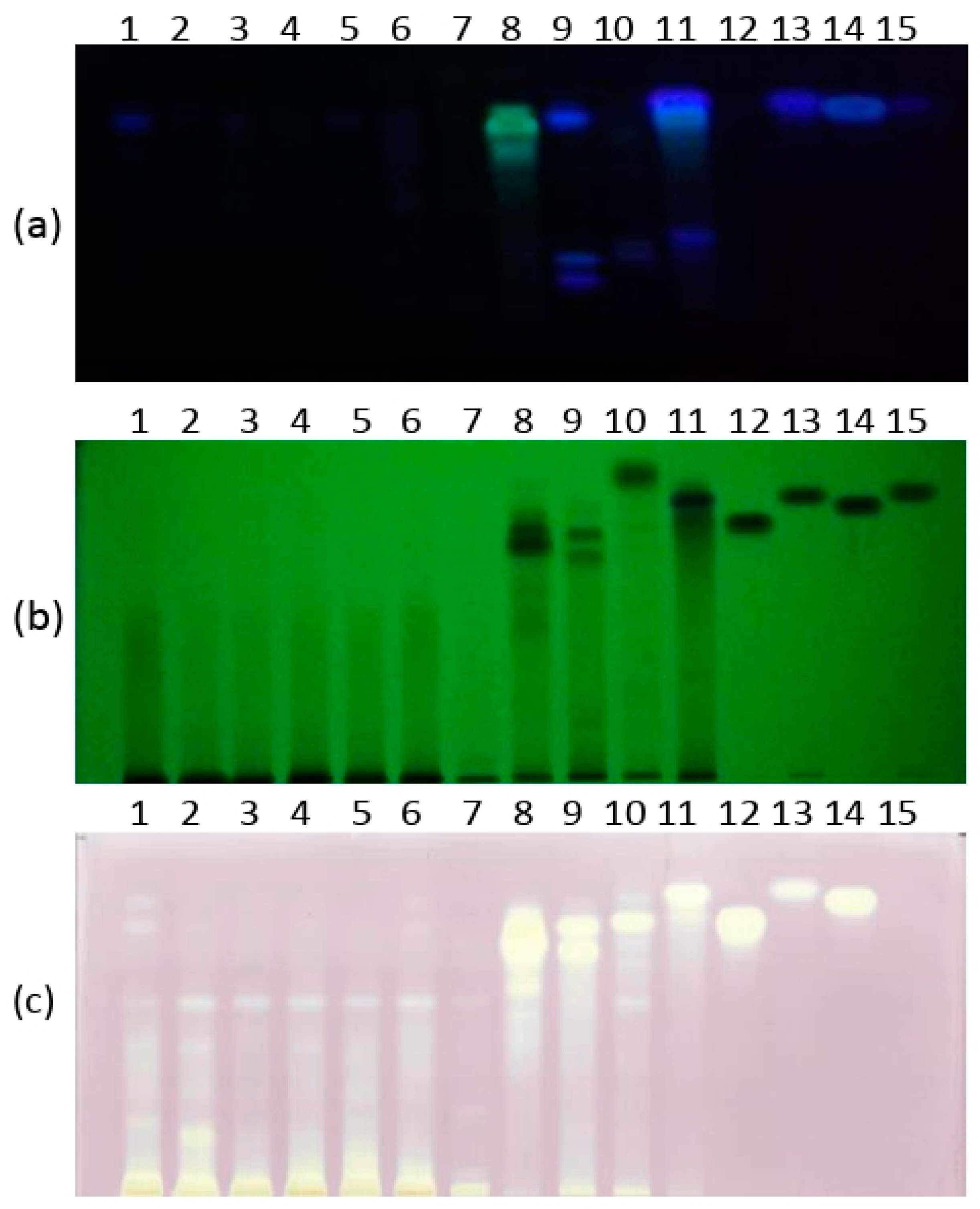

| Sample No. | Tree | Geographical Origin | Extraction Yield (%) |
|---|---|---|---|
| 1 | Pedunculate oak—Quercus robur L. | Slavonija (Croatia) | 4.44 |
| 2 | Gornji Radan (Serbia) | 4.40 | |
| 3 | Olovo (Bosnia and Herzegovina) | 4.12 | |
| 4 | Sessile oak—Quercus petraea (Matt.) Liebl. | Kučaj (Serbia) | 5.06 |
| 5 | Kuršumlija (Serbia) | 3.05 | |
| 6 | Ravna Gora (Serbia) | 4.58 | |
| 7 | Turkey oak—Quercus cerris L. | Kuršumlija (Serbia) | 1.63 |
| 8 | Black locust—Robinia pseudoacacia L. | Kraljevo (Serbia) | 6.37 |
| 9 | Myrobalan plum—Prunus cerasifera Ehrh | Vrnjačka Banja (Serbia) | 5.80 |
| 10 | Wild cherry—Prunus avium L. | Ravna Gora (Serbia) | 3.15 |
| 11 | Mulberry—Morus alba L. | Vrnjačka Banja (Serbia) | 7.29 |
| Indicator Strains | Isolate Code | Growth Medium | Growth Temperature | The Origin of The Isolates |
|---|---|---|---|---|
| Streptococcus mutans | IBR S0001 | LA | 37 °C | Oral cavity * |
| Streptococcus pyogenes | IBR S0004 | ǁ | ||
| Methicillin-resistant Staphylococcus aureus (MRSA) | ATCC33591 | ǁ | Reference strains | |
| Staphylococcus aureus | ATCC25923 | ǁ | ||
| Escherichia coli | ATCC25922 | ǁ | ||
| Enterococcus faecalis | ATCC29212 | ǁ | ||
| Listeria monocytogenes | ATCC19111 | BHA | ||
| Candida albicans | ATCC10231 | TSA |
| Indicator Strains/MIC (mg mL−1) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Str | Van | Nys |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. mutans | 0.25 | 0.13 | 0.25 | 0.25 | 0.25 | 0.25 | 0.13 | 0.25 | 1.00 | 0.05 | 0.13 | 0.020 | 0.006 | NT |
| S. pyogenes | 0.03 | 0.13 | 0.03 | 0.05 | 0.03 | 0.13 | 0.08 | 0.05 | 0.03 | 0.03 | 0.03 | 0.002 | 0.001 | NT |
| S. aureus | 0.08 | 0.05 | 0.05 | 0.03 | 0.03 | 0.03 | 0.03 | 0.09 | 0.06 | 0.05 | 0.09 | 0.009 | 0.002 | NT |
| MRSA | 0.06 | 0.06 | 0.13 | 0.06 | 0.03 | 0.05 | 0.03 | 0.03 | 0.13 | 0.13 | 0.02 | - | - | NT |
| L. monocytogenes | 0.50 | 0.50 | 0.75 | 0.75 | 0.19 | 0.63 | 0.50 | 0.13 | 0.06 | 0.06 | 0.03 | 0.019 | 0.002 | NT |
| E. coli | 0.75 | 0.75 | 1.50 | 1.50 | 0.75 | 1.50 | - | 0.75 | - | 0.75 | 0.75 | 0.009 | 0.200 | NT |
| C. albicans | - | - | - | - | - | - | - | - | 2.00 | 0.25 | - | NT | NT | 0.006 |
| Indicator strains/MBC (mg mL−1) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Str | Van | Nys |
| S. mutans | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 0.50 | 2.00 | 2.00 | 0.06 | 0.50 | 0.050 | 0.150 | NT |
| S. pyogenes | 0.50 | 0.50 | 1.00 | 1.00 | 0.50 | 0.50 | 1.00 | 0.25 | 2.00 | 0.50 | 1.00 | 0.050 | 0.050 | NT |
| S. aureus | 0.25 | 0.13 | 0.25 | 0.25 | 0.13 | 0.25 | 0.50 | 0.13 | 0.25 | 0.13 | 0.13 | 0.025 | 0.003 | NT |
| MRSA | 0.50 | 0.50 | 0.50 | 0.50 | 0.25 | 0.50 | 0.63 | 0.25 | 1.00 | 0.25 | 0.03 | - | - | NT |
| L. monocytogenes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 0.13 | 0.25 | 0.025 | 0.006 | NT |
| E. coli | 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 2.00 | - | 1.00 | - | 1.00 | 1.00 | 0.013 | 0.400 | NT |
| C. albicans | - | - | - | - | - | - | - | - | - | 0.50 | - | NT | NT | 0.025 |
| Bark Extracts Origin MIC (mg mL−1) | Sa | Mr | Lm | Sm | Sp | Ec | Ca | References |
|---|---|---|---|---|---|---|---|---|
| Picea abies | 0.13 | - | 0.16 | - | - | 0.08 | 0.97 | [37] |
| Larix decidua | 0.21 | - | 0.15 | - | - | 0.33 | 0.60 | |
| Quercus acutissima | 0.23 | - | 0.27 | - | - | 0.17 | 0.40 | [24] |
| Quercus macrocarpa | 0.22 | - | 0.29 | - | - | 0.13 | 0.34 | |
| Quercus robur | 0.23 | - | 0.25 | - | - | 0.10 | 0.31 | |
| Quercus robur | 0.08 | - | - | - | - | 0.08 | - | [38] |
| Quercus ilex | 0.13 | - | - | - | 0.51 | 0.26 | - | [39] |
| Quercus infectoria | - | 1.25 | - | - | - | - | - | [40] |
| Maclura tinctoria | - | - | - | 0.08 | - | - | - | [41] |
| Prunus africana | 0.07 | 0.16 | - | - | - | - | - | [42] |
| Prunus avium | 6.25 | - | - | - | - | 12.50 | - | [43] |
| Prunus cerasoides | 5.00 | 1.00 | - | - | - | - | 1.00 | [44] |
| Morus mesozygia | 0.16 | - | - | - | - | 0.04 | 0.16 | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smailagić, A.; Ristivojević, P.; Dimkić, I.; Pavlović, T.; Dabić Zagorac, D.; Veljović, S.; Fotirić Akšić, M.; Meland, M.; Natić, M. Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts. Foods 2020, 9, 319. https://doi.org/10.3390/foods9030319
Smailagić A, Ristivojević P, Dimkić I, Pavlović T, Dabić Zagorac D, Veljović S, Fotirić Akšić M, Meland M, Natić M. Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts. Foods. 2020; 9(3):319. https://doi.org/10.3390/foods9030319
Chicago/Turabian StyleSmailagić, Anita, Petar Ristivojević, Ivica Dimkić, Tamara Pavlović, Dragana Dabić Zagorac, Sonja Veljović, Milica Fotirić Akšić, Mekjell Meland, and Maja Natić. 2020. "Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts" Foods 9, no. 3: 319. https://doi.org/10.3390/foods9030319
APA StyleSmailagić, A., Ristivojević, P., Dimkić, I., Pavlović, T., Dabić Zagorac, D., Veljović, S., Fotirić Akšić, M., Meland, M., & Natić, M. (2020). Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts. Foods, 9(3), 319. https://doi.org/10.3390/foods9030319






