Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Passion Fruit Pulp
2.3. Growth Kinetics
2.4. Spray Drying
2.4.1. Process Yield
2.4.2. Cell Number and Encapsulation Efficiency
2.4.3. Powder Moisture Content
2.5. Statistical Analysis
3. Results and Discussions
3.1. Characterization of Passion Fruit Pulp in Natura
3.2. Lactobacillus Reuteri Growth during Passion Fruit Pulp Fermentation
3.3. Physicochemical and Phytochemical Characteristics of the Fermentative Process
3.4. Production of Probiotic Passion Fruit Powder using Spray Drying
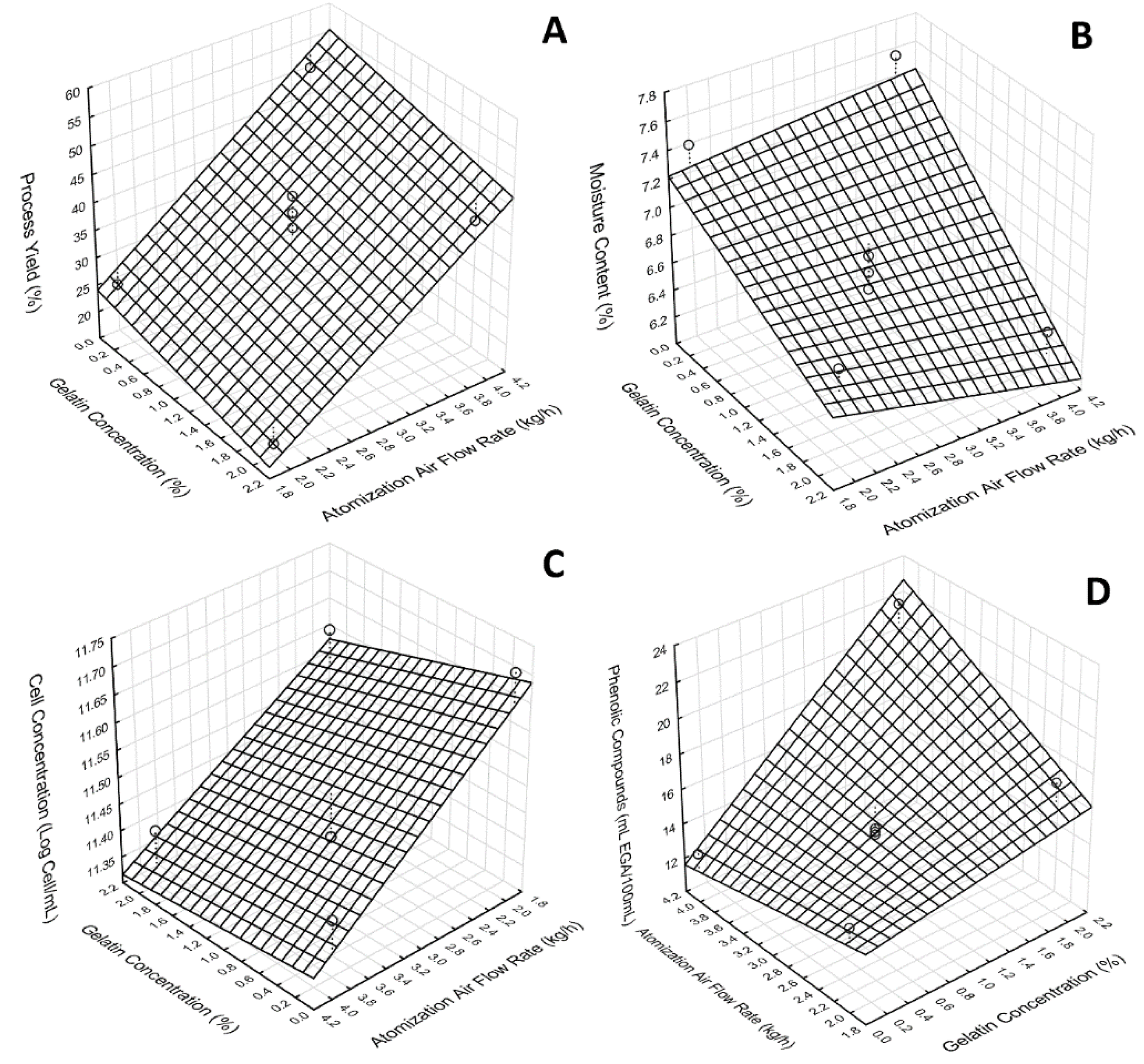
3.4.1. Concentration of Viable Cells in the Passion Fruit Probiotic Pulp
3.4.2. Process Yield
3.4.3. Phenolic Content
3.4.4. Moisture Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Binda, S.; Bron, P.A.; Gross, G.; Hill, C.; van Hylckama Vlieg, J.E.; Lebeer, S.; Satokari, R.; Ouwehand, A.C. Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr. Opin. Biotechnol. 2019, 56, 55–60. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Dias, C.O.; de Almeida, J.D.S.O.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; Amboni, R.D.D.M.C. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. 2004, 42, 315–318. [Google Scholar]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.; Pandiella, S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Rinaldi, M.M.; Costa, A.M.; Faleiro, F.G.; Junqueira, N.T.V. Conservação pós-colheita de frutos de Passiflora setacea DC. submetidos a diferentes sanitizantes e temperaturas de armazenamento. Braz. J. Food Technol. 2017, 20, 12. [Google Scholar] [CrossRef]
- Silva, E.C.O.D. Influência do Flavedo e da Maceração nas Características Físico-Químicas da Farinha da Casca de Maracujá; Universidade Federal de Campina Grande: Campina Grande, Brazil, 2017; p. 56. [Google Scholar]
- Lisboa, H.M.; Duarte, M.E.; Cavalcanti-Mata, M.E. Modeling of food drying processes in industrial spray dryers. Food Bioprod. Process. 2018, 107, 49–60. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Characterization of spray dried probiotic Sohiong fruit powder with Lactobacillus plantarum. LWT 2020, 117, 108699. [Google Scholar] [CrossRef]
- Arepally, D.; Goswami, T.K. Effect of inlet air temperature and gum Arabic concentration on encapsulation of probiotics by spray drying. LWT 2019, 99, 583–593. [Google Scholar] [CrossRef]
- Looi, Y.F.; Ong, S.P.; Julkifle, A.; Alias, M.S. Effects of pretreatment and spray drying on the physicochemical properties and probiotics viability of Moringa (Moringa oleifera Lam) leaf juice powder. J. Food Process. Preserv. 2019, 43, e13915. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz (IAL). Químicos e Físicos para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008; p. 1020.
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- A.O.A.C. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; p. 3172. [Google Scholar]
- Morais, A.; Xavier, A.; Silva, G.; Silva, M.; Pagani, A. Bioactivation of Carbonated Mineral Water with Passion Fruit Microcapsules. Int. J. Nutr. Food Sci. 2015, 4, 310–319. [Google Scholar] [CrossRef]
- del Olmo, A.; Picon, A.; Nuñez, M. Probiotic dynamics during the fermentation of milk supplemented with seaweed extracts: The effect of milk constituents. LWT 2019, 107, 249–255. [Google Scholar] [CrossRef]
- Palmfeldt, J.; Hahn-Hägerdal, B. Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. Int. J. Food Microbiol. 2000, 55, 235–238. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, W.; Zhu, X. Effect of lactose content on dielectric properties of whole milk and skim milk. Int. J. Food Sci. Technol. 2018, 53, 2037–2044. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; de Almeida Lopes, M.M.; Moura, C.F.H.; de Siqueira Oliveira, L.; de Souza, K.O.; Gomes Filho, E.; Urban, L.; de Miranda, M.R.A. Effects of organic vs. conventional farming systems on quality and antioxidant metabolism of passion fruit during maturation. Sci. Hortic. 2017, 222, 84–89. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef]
- Araújo, C.L.; Bezerra, I.W.; Dantas, I.C.; Lima, T.V.; Oliveira, A.S.; Miranda, M.R.A.; Sales, M.P. Biological activity of proteins from pulps of tropical fruits. Food Chem. 2004, 85, 107–110. [Google Scholar] [CrossRef]
- De Marchi, M.; Fagan, C.C.; O’donnell, C.; Cecchinato, A.; Dal Zotto, R.; Cassandro, M.; Penasa, M.; Bittante, G. Prediction of coagulation properties, titratable acidity, and pH of bovine milk using mid-infrared spectroscopy. J. Dairy Sci. 2009, 92, 423–432. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Liu, J.; Wang, Q.; Wang, C.; Yin, Z.; Wu, C. Lactic acid production from Sophora flavescens residues pretreated with sodium hydroxide: Reutilization of the pretreated liquor during fermentation. Bioresour. Technol. 2017, 241, 915–921. [Google Scholar] [CrossRef]
- Hernández, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.; Roos, S.; Håkansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. AMB Express 2019, 9, 66. [Google Scholar] [CrossRef]
- Alvarado, A.; Behrens, W.; Josenhans, C. Protein Activity Sensing in Bacteria in Regulating Metabolism and Motility. Front. Microbiol. 2020, 10, 3055. [Google Scholar] [CrossRef]
- Copello Rotili, M.C.; Coutro, S.; Celant, V.M.; Ariane Vorpagel, J.; Barp, F.K.; Busch Salibe, A.; Costa Braga, G. Composição, atividade antioxidante e qualidade do maracujá-amarelo durante armazenamento. Semin. Ciênc. Agrár. 2013, 34, 227–240. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Patel, A.R. Probiotic fruit and vegetable juices- recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage—ScienceDirect. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Guerin, J.; Petit, J.; Burgain, J.; Borges, F.; Bhandari, B.; Perroud, C.; Desobry, S.; Scher, J.; Gaiani, C. Lactobacillus rhamnosus GG encapsulation by spray-drying: Milk proteins clotting control to produce innovative matrices. J. Food Eng. 2017, 193, 10–19. [Google Scholar] [CrossRef]
- Rocha, A.P.T.; Lisboa, H.M.; Alsina, O.L.S.; Silva, O.S. Coating process of Phyllanthus niruri Linn granules using spouted bed. Powder Technol. 2018, 336, 85–91. [Google Scholar] [CrossRef]
- Dantas, D.; Pasquali, M.A.; Cavalcanti-Mata, M.; Duarte, M.E.; Lisboa, H.M. Influence of Spray Drying Conditions on the Properties of Avocado Powder Drink. Food Chem. 2018, 266, 284–291. [Google Scholar] [CrossRef]
- Liu, X.; Hou, C.; Zhang, J.; Zeng, X.; Qiao, S. Fermentation conditions influence the fatty acid composition of the membranes of L actobacillus reuteri I 5007 and its survival following freeze-drying. Lett. Appl. Microbiol. 2014, 59, 398–403. [Google Scholar] [CrossRef]
- Ferreira, S.; Araujo, T.; Souza, N.; Rodrigues, L.; Lisboa, H.M.; Pasquali, M.; Trindade, G.; Rocha, A.P. Physicochemical, morphological and antioxidant properties of spray-dried mango kernel starch. J. Agric. Food Res. 2019, 1, 100012. [Google Scholar] [CrossRef]
- de Oliveira, A.H.; Mata, M.E.R.M.C.; Fortes, M.; Duarte, M.E.M.; Pasquali, M.; Lisboa, H.M. Influence of spray drying conditions on the properties of whole goat milk. Dry. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Guergoletto, K.B.; Busanello, M.; Garcia, S. Influence of carrier agents on the survival of Lactobacillus reuteri LR92 and the physicochemical properties of fermented juçara pulp produced by spray drying. LWT 2017, 80, 321–327. [Google Scholar] [CrossRef]
- Nascimento, A.; Cavalcanti-Mata, M.E.; Martins Duarte, M.E.; Pasquali, M.; Lisboa, H.M. Construction of a design space for goat milk powder production using moisture sorption isotherms. J. Food Process Eng. 2019, e13228. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
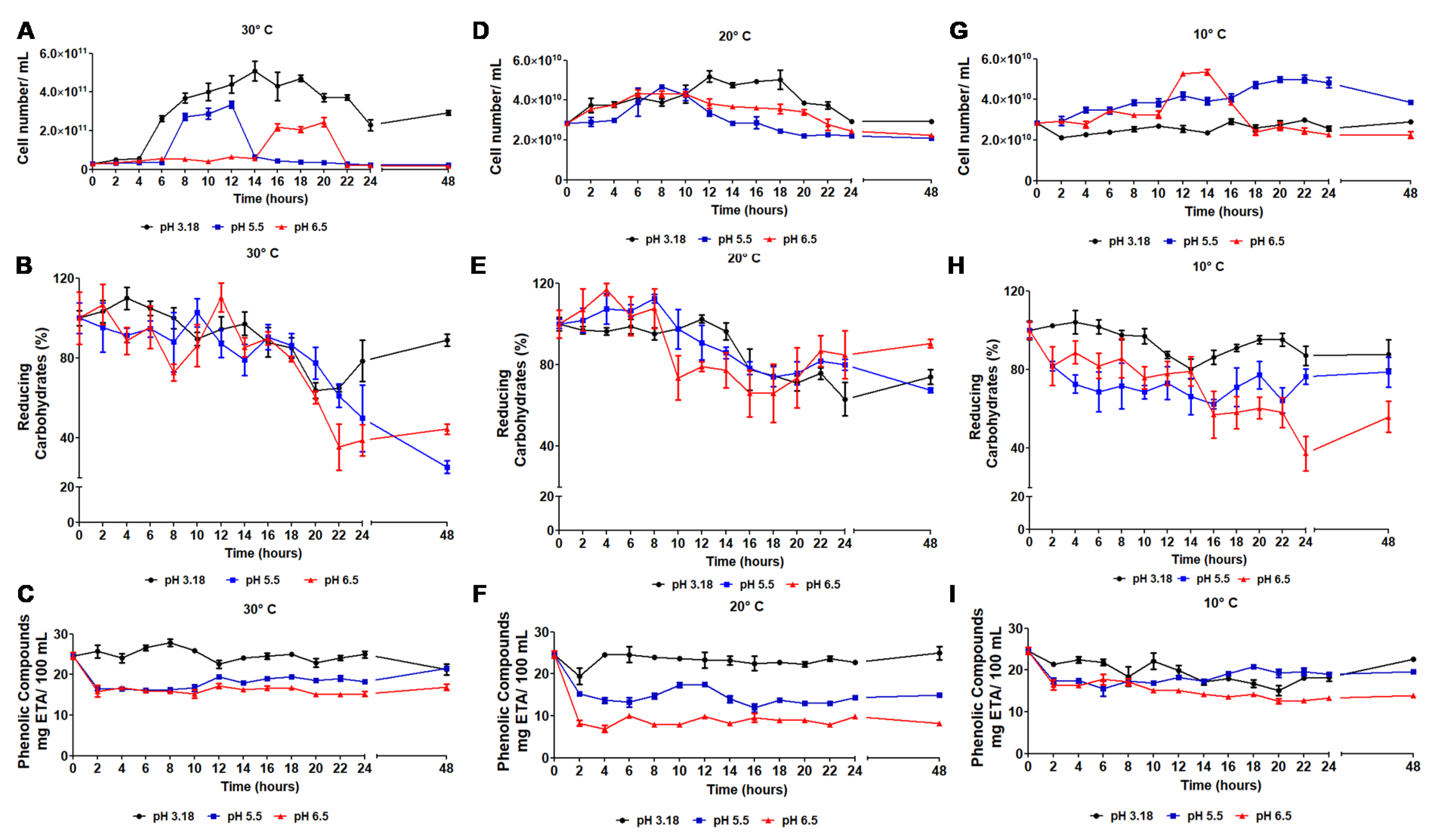


| Parameter | In Natura Pulp | 10 °C | 20 °C | 30 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.18 | 5.5 | 6.5 | 3.18 | 5.5 | 6.5 | 3.18 | 5.5 | 6.5 | ||
| pH | 3.18 ± 0.01 | 3.36 ± 0.01 | 5.61 ± 0.01 | 6.64 ± 0.01 | 2.92 ± 0.01 | 5.56 ± 0.01 | 6.22 ± 0.01 | 2.74 ± 0.01 | 5.46 ± 0.01 | 6.23 ± 0.01 |
| Total titratable acidity (%) | 4.55 ± 0.06 | 4.55 ± 0.06 | 4.55 ± 0.06 | 4.55 ± 0.06 | 4.44 ± 0.01 | 0.60 ± 0.01 | 0.12 ± 0.01 | 4.73 ± 0.05 | 0.69 ± 0.01 | 0.30 ± 0.01 |
| Total soluble solids (°Brix) | 12.33 ± 0.12 | 12.03 ± 0.03 | 12.07 ± 0.03 | 12.63 ± 0.17 | 12.27 ± 0.07 | 12.20 ± 0.12 | 12.09 ± 0.06 | 12.20 ± 0.20 | 10.40 ± 0.05 | 10.367 ± 0.07 |
| Reducing sugars (%) | 4.86 ± 0.59 | 87.11 ± 4.93 | 76.44 ± 3.97 | 37.31 ± 8.84 | 63.07 ± 8.13 | 80.03 ± 2.75 | 84.87 ± 11.78 | 78.61 ± 9.89 | 49.89 ± 9.52 | 38.91 ± 7.69 |
| Phenolic compounds (mg EGA.100 mL−1) | 36.56 ± 7.89 | 18.28 ± 1.72 | 19.06 ± 0.45 | 13.23 ± 0.21 | 22.69 ± 0.74 | 14.98 ± 0.64 | 9.91 ± 0.45 | 24.98 ± 1.45 | 18.28 ± 0.56 | 15.30 ± 1.06 |
| Proteins(mg BSA.mL−1) | 0.62 ± 0.15 | 0.66 ± 0.14 | 0.55 ± 0.16 | 0.78 ± 0.09 | 0.52 ± 0.09 | 0.54 ± 0.08 | 0.66 ± 0.05 | 1.42 ± 0.09 | 1.52 ± 0.17 | 1.43 ± 0.08 |
| Generation time (h) | NA | 76.74 | 45.61 | 12.59 | 27.99 | 7.16 | 45.69 | 2.68 | 13.40 | 23.93 |
| Max cell concentration (cells/mL) | NA | 3.01 × 1010 | 5.30 × 1010 | 5.51 × 1010 | 5.10 × 1010 | 5.29 × 1010 | 4.67 × 1010 | 5.59 × 1011 | 3.68 × 1011 | 2.55 × 1011 |
| Max growth rate (μmax h−1) | NA | 0.009 | 0.015 | 0.055 | 0.025 | 0.097 | 0.015 | 0.026 | 0.052 | 0.029 |
| Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|
| Atomization Flow tate | (kg/h) | 2.0 | 2.0 | 4.0 | 4.0 | 3.0 | 3.0 | 3.0 |
| Gelatin Concentration | (%) | 0.0 | 2.0 | 0.0 | 2.0 | 1.0 | 1.0 | 1.0 |
| Process yield | (%) | 23.6 | 17.22 | 49.67 | 41.85 | 37.32 | 42.99 | 39.94 |
| Moisture Content | (%) | 7.39 ± 0.34a | 6.71 ± 0.20ab | 7.54 ± 0.26a | 6.39 ± 0.34c | 6.53 ± 0.34c | 6.77 ± 0.32ac | 6.65 ± 0.25ac |
| Cell viability | Log cells mL−1 | 11.72 ± 0.007a | 11.62 ± 0.039b | 11.45 ± 0.034c | 11.4 ± 0.041c | 11.41 ± 0.038c | 11.41 ± 0.029c | 11.41 ± 0.007c |
| Phenolic content | mg EGA/100 mL | 14 ± 1.5a | 17 ± 2.8ab | 12.83 ± 0.15a | 22.0 ± 2.8c | 14.59 ± 0.61a | 14.24 ± 0.52a | 14.41 ± 0.54a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Monteiro, S.; Albertina Silva Beserra, Y.; Miguel Lisboa Oliveira, H.; Pasquali, M.A.d.B. Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying. Foods 2020, 9, 335. https://doi.org/10.3390/foods9030335
Santos Monteiro S, Albertina Silva Beserra Y, Miguel Lisboa Oliveira H, Pasquali MAdB. Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying. Foods. 2020; 9(3):335. https://doi.org/10.3390/foods9030335
Chicago/Turabian StyleSantos Monteiro, Shênia, Yolanda Albertina Silva Beserra, Hugo Miguel Lisboa Oliveira, and Matheus Augusto de Bittencourt Pasquali. 2020. "Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying" Foods 9, no. 3: 335. https://doi.org/10.3390/foods9030335
APA StyleSantos Monteiro, S., Albertina Silva Beserra, Y., Miguel Lisboa Oliveira, H., & Pasquali, M. A. d. B. (2020). Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying. Foods, 9(3), 335. https://doi.org/10.3390/foods9030335






