Characterization of Flavonoid Compounds in Common Swedish Berry Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Standards, and Solvents
2.2. Berry Samples
2.3. Sample Extraction
2.4. Quantification
2.5. Quality Control
2.6. Calculations and Statistical Analysis
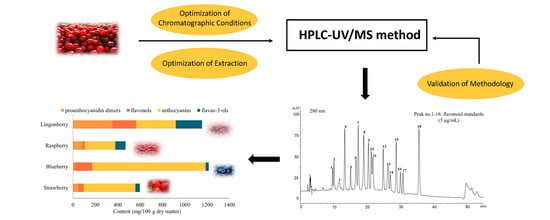
3. Results and Discussion
3.1. Method Optimization for Berry Matrix
3.2. Quality Control of Quantitative Method
3.3. Flavonoids in Swedish Berries
4. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, J.P.; Kaur, A.; Singh, N.; Nim, L.; Shevkani, K.; Kaur, H.; Arora, D.S. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci. Technol. 2016, 65, 1025–1030. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Xiao, J.B. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of insulin Resistance and Inflammation in Women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Glover, B.J.; Martin, C. Anthocyanins. Curr. Biol. 2012, 22, R147–R150. [Google Scholar] [CrossRef]
- Ou, K.Q.; Gu, L.W. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Sa, R.R.; Caldas, J.D.; Santana, D.D.; Lopes, M.V.; dos Santos, W.N.L.; Korn, M.G.A.; Santos, A.D. Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L.). Food Chem. 2019, 273, 15–23. [Google Scholar]
- Kylli, P.; Nohynek, L.; Puupponen-Pimia, R.; Westerlund-Wikstrom, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry Phenolics: Compositional Analysis and Bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.R.; Willems, J.L.; Low, N.H. Phenolic composition and antioxidant activities of saskatoon berry fruit and pomace. Food Chem. 2019, 290, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.Z.; Sinkkonen, J.; Kallio, H.; Yang, B.R. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Kajdzanoska, M.; Petreska, J.; Stefova, M. Comparison of Different Extraction Solvent Mixtures for Characterization of Phenolic Compounds in Strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Truchado, P.; Tomás-Barberán, F.A.; López-Lázaro, M.; Barradas, M.C.D.; Martín-Cordero, C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J. Food Compos. Anal. 2013, 29, 58–63. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of Extraction Methods on Phenolic Contents and Antioxidant Activity in Aerial Parts of Potentilla atrosanguinea Lodd. and Quantification of Its Phenolic Constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef]
- Pereira, G.A.; Arruda, H.S.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Carbohydrates, volatile and phenolic compounds composition, and antioxidant activity of calabura (Muntingia calabura L.) fruit. Food Res. Int. 2018, 108, 264–273. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimia, R.; Westerlund-Wikstrom, B.; Leppanen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: Isolation, Identification, and Bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.A.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Kylli, P. Berry Phenolics: Isolation, Analysis, Identification, and Antioxidant Properties. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2010. [Google Scholar]
- Bunea, A.; Rugina, D.O.; Pintea, A.M.; Sconta, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Horti Agrobot. 2011, 39, 70–76. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Latti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An Optimized Method for Analysis of Phenolic Compounds in Buds, Leaves, and Fruits of Black Currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef]
- Magalhaes, S.C.Q.; Taveira, M.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentao, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Mirali, M.; Ambrose, S.J.; Wood, S.A.; Vandenberg, A.; Purves, R.W. Development of a fast extraction method and optimization of liquid chromatography-mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 149–161. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Zafrilla, P.; Tomas-Barberan, F.A. The use of acetone as an extraction solvent for anthocyanins from strawberry fruit. Phytochem. Anal. 1998, 9, 274–277. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. The Distribution of Anthocyanins in Grape Skin Extracts of Port Wine Cultivars as Determined by High-Performance Liquid-Chromatography. J. Sci. Food Agric. 1985, 36, 1315–1324. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.M.; Martin-Ortega, G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agric. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Hajazimi, E.; Landberg, R.; Zamaratskaia, G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT-Food Sci. Technol. 2016, 74, 128–134. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Latti, A.K.; Riihinen, K.R.; Jaakola, L. Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium x intermedium Ruthe). Phytochemistry 2011, 72, 810–815. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Hellstrom, J.K.; Mattila, P.H. HPLC determination of extractable and unextractable proanthocyanidins in plant materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Sparzak, B.; Merino-Arevalo, M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Majdan, M.; Fecka, I.; Glod, D.; Baczek, T. HPLC analysis of polyphenols in the fruits of Rubus idaeus L. (Rosaceae). Nat. Prod. Res. 2010, 24, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.P.; Sonsteby, A.; Nes, A.; Wold, A.B.; Foito, A.; Freitag, S.; Verrall, S.; Stewart, D.; Heide, O.M. Effects of Post-Flowering Environmental Variation along an Altitudinal Gradient on Chemical Composition of ‘Glen Ample’ Red Raspberry (Rubus idaeus L.). Eur. J. Hortic. Sci. 2014, 79, 267–277. [Google Scholar]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Ochmian, I.; Kozos, K.; Chelpinski, P.; Szczepanek, M. Comparison of berry quality in highbush blueberry cultivars grown according to conventional and organic methods. Turk. J. Agric. For. 2015, 39, 174–181. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W.; Galletta, G.J. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar] [CrossRef] [PubMed]

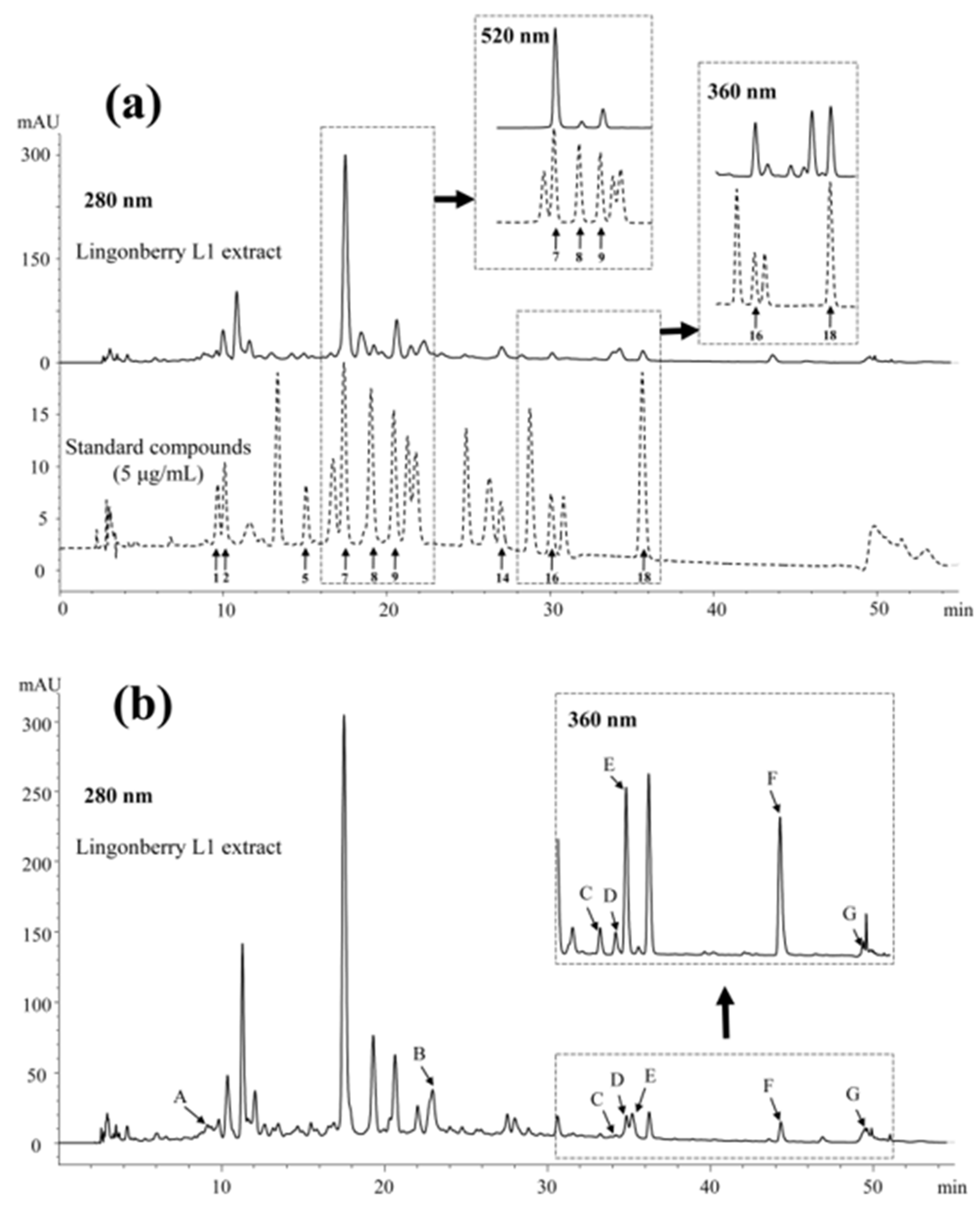

| No. | Compound | Retention Time (min) | λ 1 (nm) | Regression Equation 2 | LOD 3 (μg/mL) | LOQ 3 (μg/mL) | Abbre- Viation |
|---|---|---|---|---|---|---|---|
| 1 | Procyanidin B1 | 9.7 | 280 | y = 11.12x − 1.71 | 0.84 | 2.56 | Pr B1 |
| 2 | (+)-Catechin | 10.1 | 280 | y = 14.10x − 1.72 | 0.67 | 2.02 | (+)-Ca |
| 3 | Delphinidin-3,5-diglucoside | 11.6 | 520 | y = 22.35x − 6.10 | 0.99 | 2.99 | Del-di |
| 4 | Procyanidin B2 | 13.3 | 280 | y = 37.48x − 6.02 | 0.80 | 2.41 | Pr B2 |
| 5 | (-)-Epicatechin | 15.1 | 280 | y = 11.64x − 2.06 | 0.85 | 2.58 | (−)-Epi |
| 6 | Delphinidin-3-O-glucoside | 16.7 | 520 | y = 59.52x − 38.58 | 1.99 | 6.04 | Del-glu |
| 7 | Cyanidin-3-O-galactoside | 17.4 | 520 | y = 64.97x − 12.84 | 0.96 | 2.91 | Cy-gal |
| 8 | Cyanidin-3-O-glucoside | 19.1 | 520 | y = 57.62x − 12.71 | 1.09 | 3.30 | Cy-glu |
| 9 | Cyanidin-3-O-arabinoside | 20.5 | 520 | y = 51.51x − 11.54 | 1.11 | 3.36 | Cy-ara |
| 10 | Pelargonidin-3-O-glucoside | 21.3 | 520 | y = 31.84x − 6.29 | 0.98 | 2.96 | Pel-glu |
| 11 | Petunidin-3-O-glucoside | 21.8 | 520 | y = 56.17x − 29.61 | 1.86 | 5.64 | Pet-glu |
| 12 | Luteolin-8-C-glucoside | 24.9 | 360 | y = 53.77x − 4.83 | 0.49 | 1.49 | Lut-glu |
| 13 | Malvidin-3-O-glucoside | 26.3 | 520 | y = 48.43x − 21.34 | 1.67 | 5.06 | Mal-glu |
| 14 | Procyanidin A2 | 27.0 | 280 | y = 10.64x − 1.78 | 0.92 | 2.80 | Pr A2 |
| 15 | Myricetin-3-O-rhamnoside | 28.8 | 360 | y = 64.46x − 10.05 | 0.77 | 2.34 | My-rha |
| 16 | Quercetin-3-O-galactoside | 30.1 | 360 | y = 30.22x − 4.20 | 0.66 | 2.01 | Qu-gal |
| 17 | Quercetin-3-O-rutinoside | 30.8 | 360 | y = 28.29x − 3.76 | 0.63 | 1.90 | Qu-rut |
| 18 | Quercetin-3-O-rhamnoside | 35.6 | 360 | y = 39.95x − 6.95 | 0.89 | 2.69 | Qu-rha |
| 19 | Kaempferol-3-O-glucoside 4 | 35.6 | 360 | y = 41.27x − 19.89 | 2.16 | 6.54 | Kae-glu |
| Lingonberry 3 | Raspberry | Blueberry | Strawberry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | Kweli | Versalle | Glen Ample | Bluecrop | Duke | Camelia | Legacy | Evie | Favori | Sonata | Faith | Malwina | Salsa | Rumba | |
| Proanthocyanidins | ||||||||||||||||
| Pr B1 | 68.1 ± 0.6 | 111.6 ± 3.8 | n.d. | n.d. | n.d. | 35 ± 1.3 | 19.8 ± 0.2 | 13.9 ± 0.4 | n.d. | 12.4 ± 1.9 | 10.9 ± 0.3 | 15.2 ± 0.1 | 6 ± 0.1 | 11.9 ± 0.1 | 7.9 ± 0.7 | 12.1 ± 1.1 |
| Pr A2 | 51.9 ± 0.0 | 41.8 ± 1.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavan-3-ols | ||||||||||||||||
| (+)-Ca | 152.7 ± 2.5 | 243.6 ± 5.9 | 7.4 ± 0.1 | 6.1 ± 0.2 | 4.8 ± 0.2 | 43.1 ± 1.9 | 32 ± 0.6 | 22.6 ± 0.4 | 10.3 ± 0.6 | 38 ± 3.1 | 36.7 ± 1.3 | 45.8 ± 0.5 | 30.5 ± 1.3 | 45.4 ± 1.1 | 32 ± 1 | 48.1 ± 1 |
| (-)-Epi | 38.6 ± 0.4 | 34.1 ± 4.4 | 102.4 ± 4 | 87.1 ± 2.8 | 63.9 ± 7.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavonols | ||||||||||||||||
| My-rha | n.d. | n.d. | n.d. | n.d. | n.d. | 2.5 ± 0.1 | 2.7 ± 0.2 | 9.2 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-gal | 35.5 ± 0.0 | 58.8 ± 2.6 | 14.3 ± 0.6 | 19.6 ± 0.3 | 14.9 ± 0.4 | 78.6 ± 1.1 | 67.9 ± 1.6 | 23.7 ± 1.9 | 125.8 ± 3.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-rut | n.d. | n.d. | 4.1 ± 0.1 | 7 ± 0.2 | 9.1 ± 0.3 | 44.7 ± 2 | 32.1 ± 1.3 | 30.4 ± 2.4 | 19.9 ± 0.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-rha | 36.5 ± 0.5 | 46.1 ± 3 | n.d. | n.d. | n.d. | 1.3 ± 0.2 | 1.7 ± 0.2 | 60.2 ± 3.5 | 9.4 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kae-glu | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 9.4 ± 0.5 | 7.3 ± 0.1 | 8 ± 0.1 | 6.1 ± 0.3 | 5.3 ± 0.1 | 4.3 ± 0.1 | 10.1 ± 0.2 |
| Anthocyanidins | ||||||||||||||||
| Del-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 47 ± 2.4 | 35.1 ± 0.6 | 33.5 ± 1.4 | 5.3 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cy-gal | 308.4 ± 6.1 | 238.9 ± 5.1 | n.d. | n.d. | n.d. | 12.5 ± 0.8 | 10.5 ± 0.2 | 7.8 ± 0.5 | 20.7 ± 1.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cy-glu | 21.1 ± 0.2 | 17.1 ± 0.7 | 74.4 ± 0.8 | 65.2 ± 2.2 | 55.5 ± 2.8 | 7.7 ± 0.7 | 7.2 ± 0.2 | 3.3 ± 0.1 | n.d. | 14.3 ± 0.3 | 11.4 ± 0 | 6.5 ± 0.5 | 4.6 ± 0.1 | 5.3 ± 0.1 | 9.1 ± 0.5 | 7.2 ± 0.6 |
| Cy-ara | 75.3 ± 0.3 | 49.8 ± 0.8 | n.d. | n.d. | n.d. | 67.9 ± 3 | 47.6 ± 1.1 | 86.6 ± 4.2 | 138.4 ± 5.8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pel-glu | n.d. | n.d. | 4.5 ± 0.2 | 2.9 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | 562 ± 3.1 | 487.3 ± 6.9 | 723.9 ± 15 | 184.1 ± 7.3 | 371.4 ± 9.6 | 278.1 ± 7.1 | 353.4 ± 8 |
| Pet-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 37.7 ± 1.9 | 29.6 ± 0.9 | 33.2 ± 1.2 | 4.9 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Mal-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 94.3 ± 4.5 | 83.3 ± 1.8 | 88.6 ± 3.9 | 10.5 ± 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Compound | RT 1 (min) | [M+H] + (m/z 2) | Berry Varieties (mg/100 g dwt) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lingonberry | ||||||||||
| L1 | L2 | |||||||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin 3 | 8.8 | 579 | 46.7 ± 3 | 66 ± 6.4 | ||||||
| A-type procyanidin 4 | 22.2 | 577 | 130 ± 1.5 | 188.6 ± 5.5 | ||||||
| Flavonols 5 | ||||||||||
| Quercetin-3-O-xyloside | 32.7 | 435 | 7.1 ± 0.3 | 10.8 ± 0.5 | ||||||
| Quercetin-3-O-arabinoside | 33.7 | 435 | 6.3 ± 0.1 | 9 ± 0.4 | ||||||
| Quercetin-3-O-arabino-furanoside | 34.3 | 435 | 42.5 ± 1.9 | 56 ± 2.2 | ||||||
| Quercetin-3-O-(4’’-HMG)-rhamnoside 6 | 43.6 | 593 | 41.8 ± 1.1 | 64.3 ± 2.9 | ||||||
| Kaempferol-(HMG)-rhamnoside | 49.6 | 577 | 4.6 ± 0.1 | 3.4 ± 0.2 | ||||||
| Blueberry | ||||||||||
| Bluecrop | Duke | Camelia | Legacy | |||||||
| Anthocyanidins 7 | ||||||||||
| Delphinidin-3-O-galactoside | 15.4 | 465 | 88.8 ± 3.7 | 61.3 ± 1.4 | 110.9 ± 5.4 | 164 ± 6.8 | ||||
| Delphinidin-3-O-arabinoside | 18.4 | 435 | 87.1 ± 5.2 | 67.5 ± 1.3 | 82.3 ± 3.5 | 80.8 ± 2.9 | ||||
| Petunidin-3-O-galactoside | 20.5 | 479 | 60.8 ± 2.7 | 42.6 ± 1 | 77.5 ± 3.7 | 123.8 ± 5.1 | ||||
| Peonidin-3-O-galactoside | 23.2 | 463 | 4.4 ± 0.8 | 3.8 ± 0.2 | 2.1 ± 0.2 | n.d. | ||||
| Petunidin-3-O-arabinoside | 23.6 | 449 | 41.5 ± 2.2 | 28.9 ± 0.8 | 45.8 ± 2.2 | 47.4 ± 1.3 | ||||
| Peonidin-3-O-glucoside | 24.2 | 463 | 5.4 ± 0.7 | 6.1 ± 0.1 | 4.2 ± 0.3 | 0.4 ± 0.4 | ||||
| Malvidin-3-O-galactoside | 25.0 | 493 | 132.9 ± 6.2 | 87.9 ± 2.5 | 187.5 ± 8.7 | 289.8 ± 14 | ||||
| Peonidin-3-O-arabinoside | 25.7 | 433 | 14.2 ± 0.9 | 8.5 ± 0 | 3.7 ± 0.1 | 0.4 ± 0.4 | ||||
| Malvidin-3-O-arabinoside | 28.2 | 463 | 141.7 ± 6.1 | 95.4 ± 2.3 | 121.2 ± 6.7 | 136.5 ± 5.3 | ||||
| Delphinidin-3-acetyl-glucoside | 29.9 | 507 | 16.5 ± 0.6 | 8 ± 0 | 2.4 ± 0 | n.d. | ||||
| Petunidin-3-acetyl-glucoside | 35.0 | 521 | 15.7 ± 0.6 | 8.5 ± 0.6 | n.d. | n.d. | ||||
| Cyanidin-3-malonyl-glucoside | 35.2 | 535 | 27.7 ± 0.9 | 10.3 ± 0.4 | n.d. | n.d. | ||||
| Malvidin-3-acetyl-glucoside | 39.2 | 491 | 43.6 ± 1.4 | 19.9 ± 0.4 | 3.3 ± 0.3 | n.d. | ||||
| Flavonols 5 | ||||||||||
| Myricetin-3-O-arabinoside | 32.3 | 465 | 12.1 ± 0.6 | 10.6 ± 0.3 | n.d. | 8.5 ± 0.6 | ||||
| Quercetin-3-O-arabinoside | 33.6 | 435 | 20.2 ± 0.6 | 19.5 ± 0.7 | 7.3 ± 0.7 | 28.9 ± 1.1 | ||||
| Raspberry | ||||||||||
| Kweli | Versalle | Glen Ample | ||||||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin3 | 11.5 | 579 | 80 ± 1.6 | 123.8 ± 3.6 | 43.1 ± 4.1 | |||||
| Anthocyanidins 7 | ||||||||||
| Cyanidin-3-O-sophoroside | 17.2 | 611 | 192.4 ± 3.2 | 210.9 ± 6.5 | 81.6 ± 4.1 | |||||
| Cyanidin-3-glucosyl-rutinoside | 19.3 | 757 | n.d. | n.d. | 82.9 ± 8.9 | |||||
| Pelargonidin-3-O-rutinoside | 21.6 | 579 | n.d. | n.d. | 51.2 ± 2.5 | |||||
| Strawberry | ||||||||||
| Evie | Favori | Sonata | Faith | Malwina | Salsa | Rumba | ||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin 3 | 8.8 | 579 | 31.5 ± 2.2 | 22.7 ± 2.3 | 34.8 ± 4.6 | 57.7 ± 1.8 | 33.1 ± 1.3 | 65.8 ± 1.6 | 40.2 ± 0.3 | |
| Anthocyanidins 7 | ||||||||||
| Cyanidin-hexose-deoxyhexoside | 24.1 | 595 | 6.8 ± 0.2 | 7.9 ± 1.0 | 9.8 ± 0.2 | 5.7 ± 0.1 | 7.6 ± 0.2 | 11.8 ± 0.4 | 8.8 ± 0.3 | |
| Pelargonidin-3-O-malonylglucoside | 30.6 | 519 | 22.4 ± 0.2 | 20.3 ± 0.2 | 30.1 ± 0.9 | 23.8 ± 0.9 | 12.6 ± 0.4 | 20.3 ± 0.6 | 38.2 ± 0.8 | |
| Flavonols 5 | ||||||||||
| Quercetin-3-O-glucuronide | 30.7 | 479 | 50.7 ± 1.6 | 28.5 ± 0.3 | 21.9 ± 1.4 | 40.5 ± 1.1 | 22.8 ± 0.5 | 39.1 ± 1.7 | 47.8 ± 1.5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hefni, M.E.; Witthöft, C.M. Characterization of Flavonoid Compounds in Common Swedish Berry Species. Foods 2020, 9, 358. https://doi.org/10.3390/foods9030358
Liu J, Hefni ME, Witthöft CM. Characterization of Flavonoid Compounds in Common Swedish Berry Species. Foods. 2020; 9(3):358. https://doi.org/10.3390/foods9030358
Chicago/Turabian StyleLiu, Jiyun, Mohammed E. Hefni, and Cornelia M. Witthöft. 2020. "Characterization of Flavonoid Compounds in Common Swedish Berry Species" Foods 9, no. 3: 358. https://doi.org/10.3390/foods9030358
APA StyleLiu, J., Hefni, M. E., & Witthöft, C. M. (2020). Characterization of Flavonoid Compounds in Common Swedish Berry Species. Foods, 9(3), 358. https://doi.org/10.3390/foods9030358