Effects of Cocoa Butter and Cocoa Butter Equivalent in a Chocolate Confectionery on Human Blood Triglycerides, Glucose and Insulin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Study Protocol
2.3. Test Meal
2.4. Blood Analysis
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
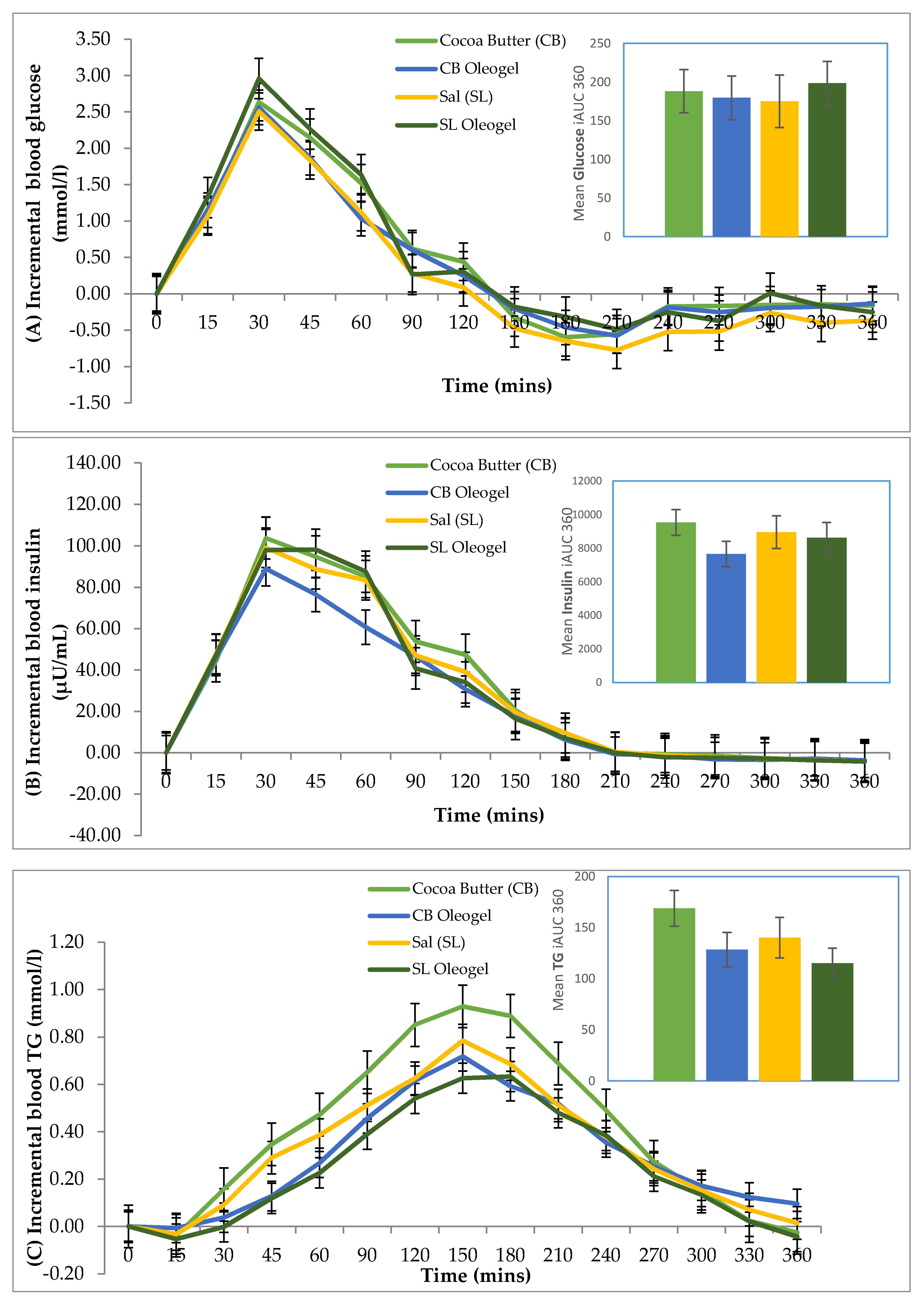
3.2. Metabolic Responses
3.3. Liking Scores of Test Meal
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Engler, M.B.; Engler, M.M. The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr. Rev. 2006, 64, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; de Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of genuine cocoa butter and cocoa butter equivalents. J. Food Compos. Anal. 2001, 14, 399–408. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Larrea, C. Global Market Report: Cocoa; International Institute for Sustainable Development: Winnipeg, MB, Canada, 2019. [Google Scholar]
- Läderach, P.; Martinez-Valle, A.; Schroth, G.; Castro, N. Predicting the future climatic suitability for cocoa farming of the world’s leading producer countries, Ghana and Côte d’Ivoire. Clim. Chang. 2013, 119, 841–854. [Google Scholar] [CrossRef]
- Lipp, M.; Anklam, E. Review of cocoa butter and alternative fats for use in chocolate—Part A. Compositional data. Food Chem. 1998, 62, 73–97. [Google Scholar] [CrossRef]
- Rios, R.V.; Pessanha, M.D.F.; Almeida PFd Viana, C.L.; Lannes, S.C.d.S. Application of fats in some food products. Food Sci. Technol. 2014, 34, 3–15. [Google Scholar] [CrossRef]
- Bloomer, S.; Adlercreutz, P.; Mattiasson, B. Triglyceride interesterification by lipases. 1. Cocoa butter equivalents from a fraction of palm oil. J. Am. Oil Chem. Soc. 1990, 67, 519–524. [Google Scholar] [CrossRef]
- Undurraga, D.; Markovits, A.; Erazo, S. Cocoa butter equivalent through enzymic interesterification of palm oil midfraction. Process Biochem. 2001, 36, 933–939. [Google Scholar] [CrossRef]
- Zaidul, I.; Norulaini, N.N.; Omar, A.M.; Smith, R., Jr. Blending of supercritical carbon dioxide (SC-CO2) extracted palm kernel oil fractions and palm oil to obtain cocoa butter replacers. J. Food Eng. 2007, 78, 1397–1409. [Google Scholar] [CrossRef]
- Chhibber, V.; Joshi, H.C.; Saxena, S.K. Sal (Shorea robusta), an Environment friendly and ecofriendly alternative vegetable oil fuel in comparison to diesel oil. Adv. Pure Appl. Chem. 2012, 1, 36–39. [Google Scholar]
- Non Timber Forest Product, Enterprise and Forest Governance. Available online: https://pdfs.semanticscholar.org/945d/6fd2a080f017f3b42cf4da84e16b7ebe7678.pdf (accessed on 2 March 2019).
- Kumar, S.; Pradhan, R.C.; Mishra, S. Exploration of Shorea robusta (Sal) seeds, kernels and its oil. Cogent Food Agric. 2016, 2, 1186140. [Google Scholar]
- Silveira, M.P.W. Research and Development: Linkages to Production in Developing Countries; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Stortz, T.A.; Zetzl, A.K.; Barbut, S.; Cattaruzza, A.; Marangoni, A.G. Edible oleogels in food products to help maximize health benefits and improve nutritional profiles. Lipid Technol. 2012, 24, 151–154. [Google Scholar] [CrossRef]
- Kheiri, M. Formulation, evaluation and marketing of cocoa butter replacer fats [palm oil products for chocolate based products]. Palm Oil Res. Inst. Malays. Occas. Paper 1982, 1, 1–53. [Google Scholar]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288. [Google Scholar] [CrossRef]
- Gammone, M.A.; Efthymakis, K.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Riccioni, G.; D’Orazio, N. Impact of Chocolate on the Cardiovascular Health. Front Biosci. (Landmark Ed) 2018, 23, 852–864. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Rolls, B.J. The role of energy density in the overconsumption of fat. J. Nutr. 2000, 130, 268S–271S. [Google Scholar] [CrossRef]
- Xiao, C.; Giacca, A.; Carpentier, A.; Lewis, G.F. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 2006, 49, 1371–1379. [Google Scholar] [CrossRef]
- Aguirre-Mandujano, E.; Lobato-Calleros, C.; Beristain, C.; Garcia, H.; Vernon-Carter, E. Microstructure and viscoelastic properties of low-fat yoghurt structured by monoglyceride gels. LWT-Food Sci. Technol. 2009, 42, 938–944. [Google Scholar] [CrossRef]
- Cadena, R.; Cruz, A.; Faria, J.; Bolini, H. Reduced fat and sugar vanilla ice creams: Sensory profiling and external preference mapping. J. Dairy Sci. 2012, 95, 4842–4850. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Peh, E.W.-Y.; Marangoni, A.G.; Henry, C.J. Effects of liquid oil vs. oleogel co-ingested with a carbohydrate-rich meal on human blood triglycerides, glucose, insulin and appetite. Food Funct. 2017, 8, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zhang, Y.; He, W.; Chen, X.; Chen, J.; He, L.; Mao, L.; Wu, F.; Jiao, J. Dietary Fats in Relation to Total and Cause-Specific Mortality in a Prospective Cohort of 521 120 Individuals With 16 Years of Follow-Up. Circ. Res. 2019, 124, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F.; Neuman, A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef] [PubMed]
- Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998, 66, 1–140.
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A. The gelation of oil using ethyl cellulose. Carbohydr. Polym. 2015, 117, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Björck, I.; Frayn, K.N.; Gibbs, A.L.; Lång, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, D.J.A. The use of the glycemie Index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Peh, E.W.Y.; Lau, E.; Marangoni, A.G.; Henry, C.J. Physical Form of Dietary Fat Alters Postprandial Substrate Utilization and Glycemic Response in Healthy Chinese Men. J. Nutr. 2017, 147, 1138–1144. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bhattacharyya, D. Enzymatic acidolysis of sal fat and its fraction. Oleagineux (France) 1991, 46, 509–513. [Google Scholar]
- Petrauskaitè, V.; De Greyt, W.F.; Kellens, M.J. Physical refining of coconut oil: Effect of crude oil quality and deodorization conditions on neutral oil loss. J. Am. Oil Chem. Soc. 2000, 77, 581–586. [Google Scholar] [CrossRef]
- Syed, A. Specialty/GM Vegetable Oils: Properties and Applications in Food (Rapeseed, Sunflower, Soybean). In Specialty Oils and Fats in Food and Nutrition; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–205. [Google Scholar]
- Kamel, B.S.; Dawson, H.; Kakuda, Y. Characteristics and composition of melon and grape seed oils and cakes. J. Am. Oil Chem. Soc. 1985, 62, 881–883. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Peh, E.W.Y.; Siow, P.C.; Marangoni, A.G.; Henry, C.J. Effects of the physical-form and the degree-of-saturation of oil on postprandial plasma triglycerides, glycemia and appetite of healthy Chinese adults. Food Funct. 2017, 8, 4433–4440. [Google Scholar] [CrossRef] [PubMed]
- Slavova, G. Global and domestic bulgarian production of cocoa and chocolate articles for the period 2013-2016. Trakia J. Sci. 2017, 15, 10–17. [Google Scholar] [CrossRef]
- Beckett, S.T. Industrial Chocolate Manufacture and Use; John Wiley & Sons: Chichester, West Sussex, UK, 2011. [Google Scholar]
- Läderach, P.; Eitzinger, A.; Martinez, A.; Castro, N. Predicting the Impact of Climate Change on the Cocoa-Growing Regions in Ghana and Côte d’Ivoire (Final Report); Climate Change Agriculture and Food Security, International Centre for Tropical Agriculture CIAT: Managua, Nicaragua, 2011; pp. 1–355. [Google Scholar]
- Schroth, G.; Läderach, P.; Dempewolf, J.; Philpott, S.M.; Haggar, J.; Eakin, H.; Castillejos, T.; Moreno, J.G.; Soto-Pinto, L.; Hernández, R.; et al. Towards a climate change adaptation strategy for coffee communities and ecosystems in the Sierra Madre de Chiapas, Mexico. Mitig. Adapt. Strat. Glob. Chang. 2009, 14, 605–625. [Google Scholar] [CrossRef]
| Characteristic | Mean ± SD |
|---|---|
| Age (years) | 24.73 ± 2.63 |
| Height (m) | 173.81 ± 7.24 |
| Weight (kg) | 65.85 ± 8.06 |
| BMI (kg/m2) | 21.73 ± 1.65 |
| Waist circumference (cm) | 78.58 ± 9.48 |
| Hip circumference (cm) | 91.03 ± 20.21 |
| Tricep skinfold (mm) | 17.85 ± 7.83 |
| Bicep skinfold (mm) | 15.40 ± 10.25 |
| Body fat (%) | 16.52 ± 5.51 |
| Systolic blood pressure (mmHg) | 119.88 ± 6.90 |
| Diastolic blood pressure (mmHg) | 74.94 ± 8.13 |
| Type of Oil | Saturated | Monounsaturated | Polyunsaturated | |||
|---|---|---|---|---|---|---|
| Lauric Acid (C12:0) | Myristic Acid (C14:0) | Palmitic Acid (C16:0) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | |
| Cocoa Butter [5] | 25.1 | 36.4 | 34.1 | 2.8 | ||
| Sal oil [33] | 6.3 | 44.6 | 41.6 | 1.7 | ||
| Coconut oil [34] | 46.6 | 18.6 | 9.5 | 2.7 | 7.0 | 1.9 |
| Palm oil [35] | 45.0 | 4.0 | 40.0 | 10.0 | ||
| Rice bran oil [36] | 22.0 | 3.0 | 38.0 | 35.0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quek, R.Y.C.; Peh, E.W.Y.; Henry, C.J. Effects of Cocoa Butter and Cocoa Butter Equivalent in a Chocolate Confectionery on Human Blood Triglycerides, Glucose and Insulin. Foods 2020, 9, 455. https://doi.org/10.3390/foods9040455
Quek RYC, Peh EWY, Henry CJ. Effects of Cocoa Butter and Cocoa Butter Equivalent in a Chocolate Confectionery on Human Blood Triglycerides, Glucose and Insulin. Foods. 2020; 9(4):455. https://doi.org/10.3390/foods9040455
Chicago/Turabian StyleQuek, Rina Yu Chin, Elaine Wan Yi Peh, and Christiani Jeyakumar Henry. 2020. "Effects of Cocoa Butter and Cocoa Butter Equivalent in a Chocolate Confectionery on Human Blood Triglycerides, Glucose and Insulin" Foods 9, no. 4: 455. https://doi.org/10.3390/foods9040455
APA StyleQuek, R. Y. C., Peh, E. W. Y., & Henry, C. J. (2020). Effects of Cocoa Butter and Cocoa Butter Equivalent in a Chocolate Confectionery on Human Blood Triglycerides, Glucose and Insulin. Foods, 9(4), 455. https://doi.org/10.3390/foods9040455





