Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy
Abstract
:1. Introduction
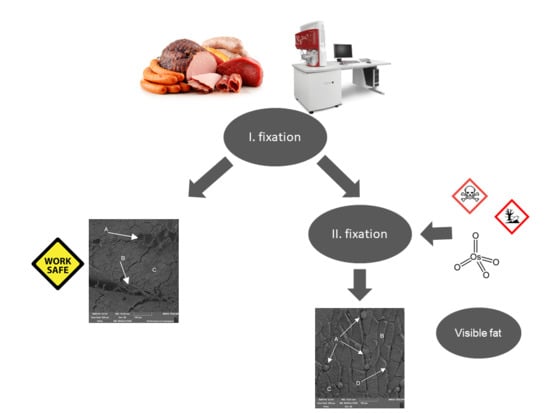
2. Material and Method
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Český statistický úřad. Available online: https://www.czso.cz/csu/czso/spotreba-potravin-2018 (accessed on 29 December 2019).
- Statista. Available online: https://www.statista.com/statistics/679528/per-capita-meat-consumption-european-union-eu/ (accessed on 29 December 2019).
- OECD. Meat Consumption (Indicator). 2019. Available online: https://doi.org/10.1787/fa290fd0-en (accessed on 29 December 2019).
- FAO. Decree on Requirements for Meat, Meat Product, Fishery and Aquaculture Products and Products Thereof, Eggs and Products Thereof; Ministry of Agriculture: Prague, Czech Republic, 2016. [Google Scholar]
- Nowak, J.D.; Rzepiejewska-Malyska, K.A.; Major, R.C.; Warren, O.L.; Michler, J. In-situ nanoindentaiton in the SEM. Mater. Today 2010, 12, 44–45. [Google Scholar] [CrossRef]
- Tafti, A.P.; Kirkpatrick, A.B.; Alavi, Z.; Owen, H.A.; Yu, Z. Recent advances in 3D SEM surface reconstruction. Micron 2015, 78, 54–66. [Google Scholar] [CrossRef]
- Moran, P.; Coats, B. Biological Sample Preparation for SEM Imaging of Porcine Retina. Microsc. Today 2012, 20, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.B., Jr.; Safiejko-Mroczka, B. Preparing whole mounts of biological specimens for imaging macromolecular structures by light and electron microscopy. Int. J. Imaging Syst. Technol. 1997, 8, 225–239. [Google Scholar] [CrossRef]
- Bahr, G.F.; Bloom, G.; Friberg, U. Volume changes of tissue in physiological fluids during fixation in osmium tetroxide or fermaldehyde and during subsequent treatment. Exp. Cell Res. 1957, 12, 342–355. [Google Scholar] [CrossRef]
- Wollweber, L.; Stracke, R.; Gothe, U. The use of a simple method to avoid cell shrinkage during SEM preparation. J. Microsc. 1981, 121, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bruk, U.; Collins, V.P.; Arro, P. The fixation, dehydration, drying and coating of cultured cells for SEM. J. Microsc. 1981, 123, 121–131. [Google Scholar] [CrossRef]
- Hopwood, D. Fixatives and fixation: A review. Histochem. J. 1969, 1, 323–360. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for SEM of plant surface. Mater. Today 2010, 12, 32–43. [Google Scholar] [CrossRef]
- Migneault, I.; Dartuquenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyd: Behavior in aquaeous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Kashi, A.M.; Tahermanesh, K.; Chaichian, S.; Joghataei, M.T.; Moradi, F.; Tavangar, S.M.; Najafabadi, A.S.M.; Lotfibakhshaiesh, N.; Beyranvand, S.P.; Anvari-Yazdi, A.F.; et al. How to Prepare Biological Samples and Live Tissue for Scanning Electron Microscopy (SEM). Galen Med. J. 2014, 3, 63–80. [Google Scholar]
- FAO. Decree on Collection, Preparation and Testing Methods of Control Samples of Food and Tobacco Products; Ministry of Agriculture: Prague, Czech Republic, 2016. [Google Scholar]
- Brooker, B.E. The Stabilisation of Air in Foods Containig Fat—A Rewiew. Food Struct. 1993, 12, 115–122. [Google Scholar]
- Kiernanm, J.A. Feromaldehyde, formalin, paraformaldehyde and glutaraldehyde: What they are and what they do. Microsc. Today 2000, 8, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Nebesářová, J. Elektronová Mikroskopie pro Biology. 2001. Available online: http://triton.paru.cas.cz/old-lem/book/index.html (accessed on 29 December 2019).
- Fisher, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protocol. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar]
- Gordon, A.; Barbut, S. Cold Stage Scanning Electron Microscopy Study of Meat Batters. J. Food Sci. 1990, 55, 1196–1198. [Google Scholar] [CrossRef]
- Kalab, M. Artedacts in Conventional Scanning Electron Microscopy of Some Milk Products. J. Food Struct. 1984, 3, 95–111. [Google Scholar]
- Li, Y.; Almassalha, L.M.; Chandler, J.E.; Zhou, X.; Stypula-Cyrus, Y.E.; Hujsak, K.A.; Roth, E.W.; Bleher, R.; Subramanian, H.; Szleifer, I.; et al. The effect of chemical fixation on the cellular nanostructure. Exp. Cell Res. 2017, 358, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Hopwood, D. Theoretical and practical aspects of glutaraldehyde fixation. In Fixation in Histochemistry; Stoward, P.J., Ed.; Springer: Boston, MA, USA, 1973. [Google Scholar]
- Gil, J.; Weibel, E.R. The role of buffers in lung fixation with glutaraldehyde and osmium tetroxide. J. Ultra Res. 1968, 25, 331–348. [Google Scholar] [CrossRef]
- Amerine, M.A.; Pangborn, R.M.; Roessler, E.B. Principles of Senzory Evaluation of Food; Academic Press Inc.: London, UK, 1965. [Google Scholar]
- Egan, A.F. Lactic acid bacteria of meat products. Antomie van Leeuwenhoek 1983, 49, 327–336. [Google Scholar] [CrossRef]
- Leroy, F.; Verluten, J.; Vuyst, L.D. Functional meat starter for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Hong, S.I.; Pyun, Y.R. Inactivation Kinetics of Lactobacillus plantarum by High Pressure Carbon Dioxide. J. Food Sci. 1999, 64, 728–733. [Google Scholar] [CrossRef]
- Venkateshwari, S.; Halami, P.M.; Vijayendra, S.V.N. Characterisation of the heat-stable bacteriocin-producting and vancomycin-sensitive Pediococcus pentosaceus CFR B19 isolated from beans. Benef. Microbes 2010, 1, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lücke, F.-K. Fermented meat products. Food Res. Int. 1994, 24, 299–307. [Google Scholar] [CrossRef]
- Murakami, T. A Metal Impregnation Method of Biological Specimens for Scanning Electron Microscopy. Arch. Histol. Jap. 1973, 35, 323–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia, J.C.; Kasthuri, N.; Hayworth, K.; Schalek, R.; Lichtman, J.W.; Smith, S.J.; Buchanan, J. High contrast en bloc staining of neuronal tissue for field emission scanning electron microscopy. Nat. Protoc. 2012, 7, 193–206. [Google Scholar] [CrossRef]
- Murakami, T.; Unehira, M.; Kawakami, H.; Kubotsu, A. Osmium Impregnation of Methyl Methacrylate Vascular Costs for Scanning Electron Microscopy. Arch. Histol. Jap. 1973, 36, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Apkarian, R.P.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron. Microscopy (SEM). Scanning Microscopy for Nanotechnology; Springer: New York, NY, USA, 2006; pp. 1–40. [Google Scholar]
- Deerinck, T.; Bushong, E.; Lev-Ram, V.; Shu, X.; Tsien, R.; Ellisman, M. Enhancing Serial Block-Face Scanning Electron Microscopy to Enable High Resolution 3-D Nanohistology of Cells and Tissues. Microsc. Microanal. 2010, 16, 1138–1139. [Google Scholar] [CrossRef] [Green Version]
- Goizueta, G.; Chiba, T.; Inoue, T. Phase morphology of polymer blends: Scanning electron microscope observation by backscattering from a microtomed and stained surface. Polymer 1992, 33, 886–888. [Google Scholar] [CrossRef]









| Chemicals | Concentration | Time | Procedure 1 | Procedure 2 |
|---|---|---|---|---|
| glutaraldehyde | 3% | 24 h | x | x |
| buffer-cacodylate | 3 × 15 min | x | ||
| osmium tetroxide | 1% | 48 h | x | |
| buffer-cacodylate | 3 × 15 min | x | ||
| ethanol of increasing concentrations | 25%, 50%, 70%, 85%, 90%, 96%, 100% | every 15 min | x | x |
| Type of Meat Product | Fixation Method | Containing Muscle with Visible Muscle Fibers | Space between Muscle Fibers | Shape of Muscle Fibers on Crosscut | Connective Tissue | Adipose Cells | Homogeneity | Artifacts | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Non-heat treated | 1 * | x | x | x | x | x | x | x | x |
| x | x | x | x | x | x | x | x | ||
| 2 ** | x | x | x | x | x | x | x | x | |
| x | x | x | x | x | x | x | x | ||
| Cans | 1 | ✓ | x | x | x | ✓ | +++ | x | modified starch, air gaps |
| x | x | x | x | x | +++ | x | air gaps | ||
| 2 | ✓ | + | oval | x | ✓ | ++ | air gaps, protein net | ||
| x | x | x | x | x | ++ | ✓ | protein net, modified starch | ||
| Heat-treated | 1 | ✓ | + | round | ✓ | ✓ | ++ | ✓ | x |
| ✓ | ++ | round | ✓ | x | ++ | x | x | ||
| 2 | ✓ | + | oval | ✓ | ✓ | ++ | ✓ | x | |
| ✓ | ++ | oval | ✓ | x | ++ | x | |||
| Long-life heat-treated | 1 | x | x | x | x | ✓ | +++ | ✓✓ | air gaps |
| ✓ | x | x | x | x | +++ | x | air gaps | ||
| 2 | ✓ | oval | ✓ | ✓ | ++ | ✓ | air gaps, crystals | ||
| x | x | x | x | x | x | x | x | ||
| Long-life fermented | 1 | ✓ | + | oval | x | ✓ | ++ | ✓✓ | cross-linking of proteins, spices-garlic, paprika |
| ✓ | + | oval | x | ++ | ✓ | LAB, protein matrix-connection, spices-paprika | |||
| 2 | ✓ | + | oval | ✓ | x | + | x | air gaps/lacunes | |
| ✓ | x | x | ✓ | ✓ | + | ✓ | LAB | ||
| Semi-preserves | 1 | ✓ | + | oval | ✓ | ✓ | ++ | ✓ | x |
| ✓ | + | round | ✓ | x | + | x | spices-caraway, paprika |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Běhalová, H.; Tremlová, B.; Kalčáková, L.; Pospiech, M.; Dordevic, D. Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy. Foods 2020, 9, 487. https://doi.org/10.3390/foods9040487
Běhalová H, Tremlová B, Kalčáková L, Pospiech M, Dordevic D. Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy. Foods. 2020; 9(4):487. https://doi.org/10.3390/foods9040487
Chicago/Turabian StyleBěhalová, Hana, Bohuslava Tremlová, Ludmila Kalčáková, Matej Pospiech, and Dani Dordevic. 2020. "Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy" Foods 9, no. 4: 487. https://doi.org/10.3390/foods9040487
APA StyleBěhalová, H., Tremlová, B., Kalčáková, L., Pospiech, M., & Dordevic, D. (2020). Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy. Foods, 9(4), 487. https://doi.org/10.3390/foods9040487