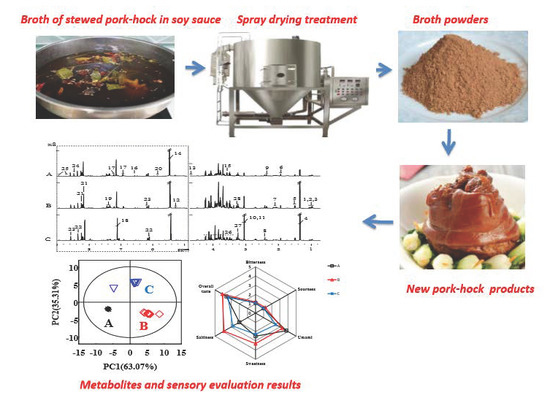
Effect of Reconstituted Broth on the Taste-Active Metabolites and Sensory Quality of Stewed and Roasted Pork-Hock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing of Broth
2.2. Spray Drying of Broth
2.3. Processing of SPHSS, SPH and MRPH
2.4. Metabolite Extraction
2.5. NMR Analysis
2.6. Data Analysis
2.7. Sensory Evaluation
3. Results and Discussion
3.1. H NMR Spectra of Extracts from SPHSS, SPH and MRPH
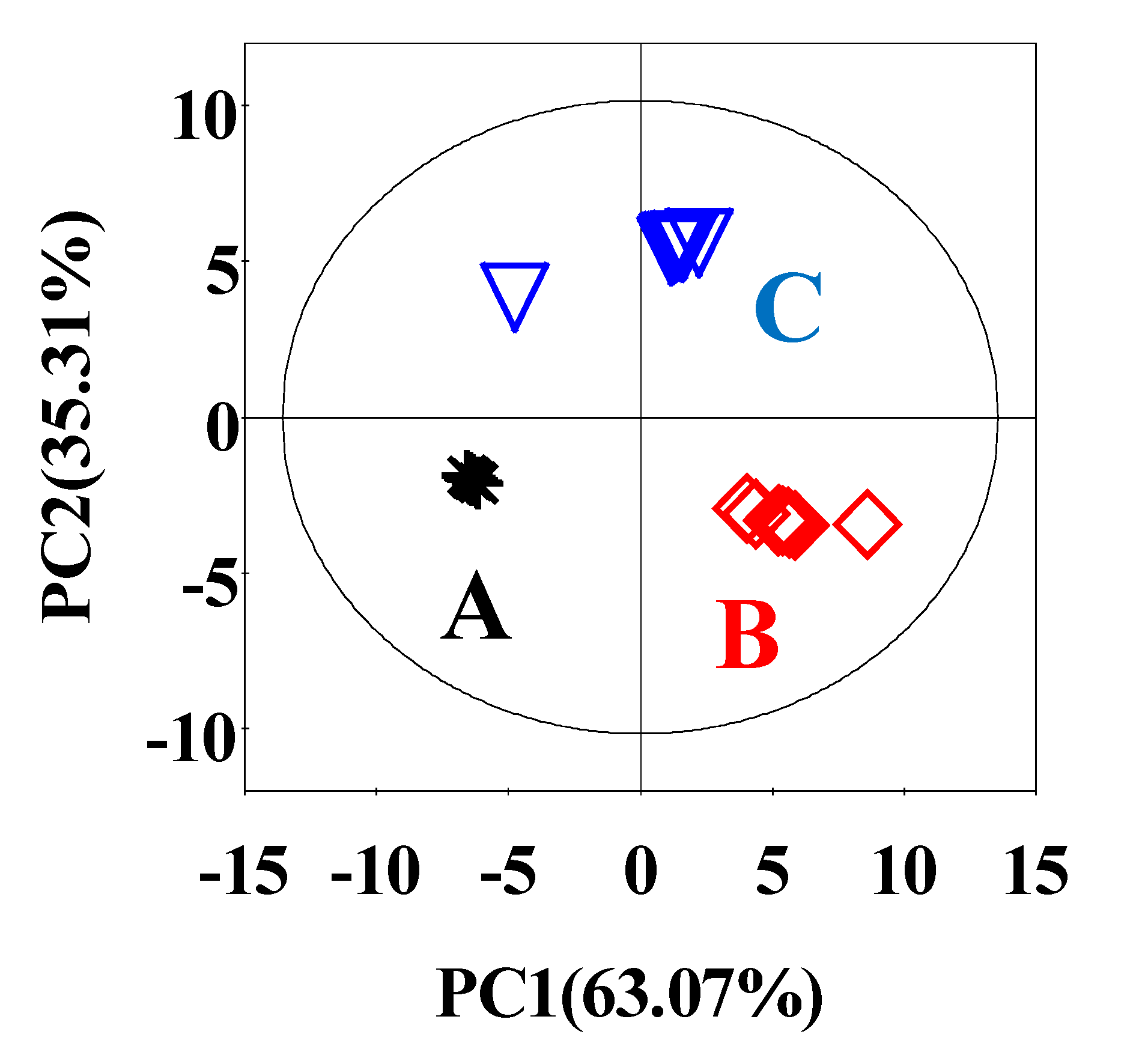
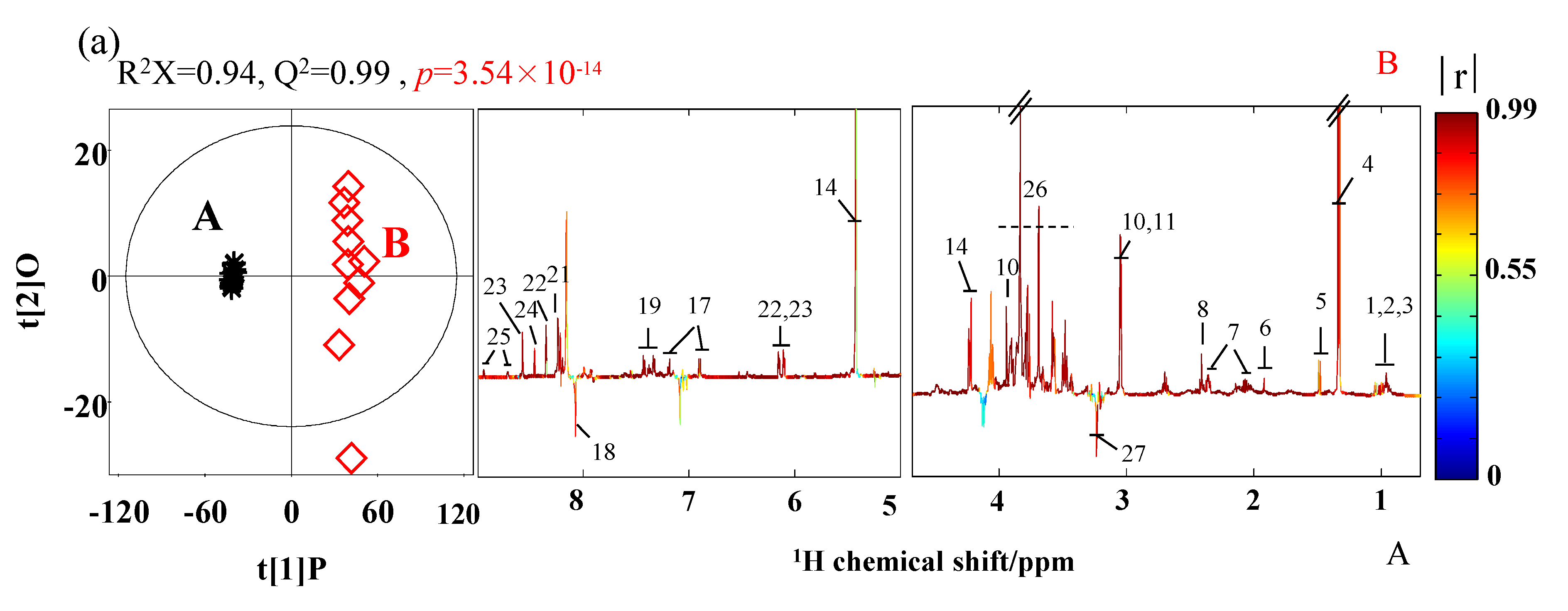
3.2. The Analysis of Metabolites of SPHSS, SPH and MRPH
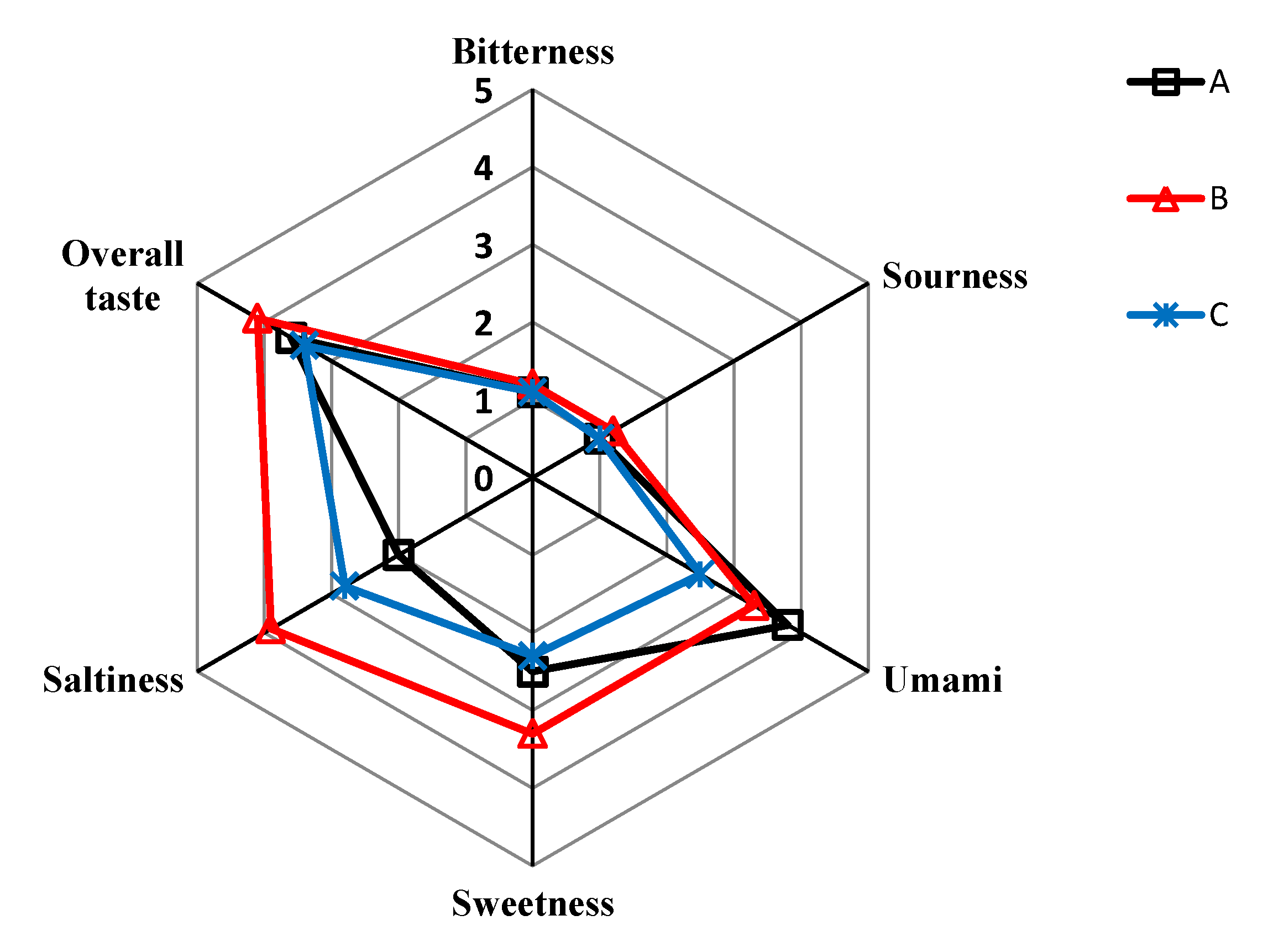
3.3. Sensory Evaluation of SPHSS, SPH and MRPH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Pan, D.; Sun, Y.; Wang, Y.; Xu, F.; Cao, J. 1H NMR-based metabolomics profiling and taste of stewed pork-hock in soy sauce. Food Res. Int. 2019, 121, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Zhou, G. Changes in taste compounds of duck during processing. Food Chem. 2007, 102, 22–26. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Orunaconcha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour: I. Effect of diet and breed. Meat Sci. 2008, 79, 124–130. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Y.; Sun, Y.; Pan, D.; Ou, C.; Dang, Y.; Wang, Y.; Cao, J.; Wang, D. 1H NMR and multivariate data analysis of the differences of metabolites in five types of dry-cured hams. Food Res. Int. 2018, 113, 140–148. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Pan, D.; Sun, Y.; Wang, Y.; Cao, J. Metabonomics profiling of marinated meat in soy sauce during processing. J. Sci. Food Agricult. 2017, 98, 1325–1331. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhao, G.M.; Liu, Y.X.; Miao-Yun, L.I.; Huang, X.Q.; Sun, L.X. Changes of free amino acids in chicken and its broth during cooking. Sci. Technol. Food Ind. 2014, 35, 333–337. [Google Scholar]
- Zhang, J.; Chen, D.; Kezong, Q.I.; Yanqiu, X.U. Determinaton of Mineral Elements in Chicken Soup by ICP-AES Method. Guangdong Trace Elements Sci. 2011, 18, 47–51. [Google Scholar]
- Qi, J.; Liu, D.Y.; Zhou, G.H.; Xu, X.L. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef]
- Chegini, G.R.; Ghobadian, B. Effect of spray-drying conditions on physical properties of orange juice powder. Drying Technol. 2005, 23, 657–668. [Google Scholar] [CrossRef]
- Islam, M.Z.; Kitamura, Y.; Yamano, Y.; Kitamura, M. Effect of vacuum spray drying on the physicochemical properties, water sorption and glass transition phenomenon of orange juice powder. J. Food Eng. 2015, 169, 131–140. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar]
- ISO 13299. Sensory Analysis-Methodology-General Guidance to Establish a Sensory Profile; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Dang, Y.L.; Wang, Z.; Xu, S.Y. Methods for extracting the taste compounds from water soluble extract of Jinhua ham. Eur. Food Res. Technol. 2008, 228, 93–102. [Google Scholar]
- Leksrisompong, P.P.; Lopetcharat, K.; Guthrie, B.; Drake, M.A. Descriptive analysis of carbonated regular and diet lemon-lime beverages. J. Sens. Stud. 2012, 27, 247–263. [Google Scholar]
- Zhang, J.; Yao, Y.; Ye, X.; Fang, Z.; Chen, J.; Wu, D.; Liu, D.; Hu, Y. Effect of cooking temperatures on protein hydrolysates and sensory;quality in crucian carp (Carassius auratus) soup. J. Food Sci. Technol. 2013, 50, 542–548. [Google Scholar]
- Kamal, G.M.; Yuan, B.; Hussain, A.I.; Wang, J.; Jiang, B.; Zhang, X.; Liu, M. 13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces. Molecules 2016, 21, 1168. [Google Scholar]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. J. Food Sci. 2016, 80, C2711–C2716. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89 Pt 1, 39–47. [Google Scholar] [CrossRef]
- Pérezpalacios, T.; Eusebio, J.; Palma, S.F.; Carvalho, M.J.; Mirbel, J.; Antequera, T. Taste compounds and consumer acceptance of chicken soups as affected by cooking conditions. Int. J. Food Prop. 2017, 20, S154–S165. [Google Scholar]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste enhancements between various amino acids and IMP. Chem. Sens. 2002, 27, 739. [Google Scholar]
- Breslin, P.A. Interactions among salty, sour and bitter compounds. Trends Food Sci. Technol. 1996, 7, 390–399. [Google Scholar]
- Grajales, A. Effect of lactic acid on the meat quality properties and the taste of pork Serratus ventralis muscle. Agricult. Food Sci. 2015, 21, 171–181. [Google Scholar]
- Kim, Y.H.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168. [Google Scholar]
- Shah, A.K.M.A.; Ogasawara, M.; Egi, M.; Kurihara, H.; Takahashi, K. Identification and sensory evaluation of flavour enhancers in Japanese traditional dried herring (Clupea pallasii) fillet. Food Chem. 2010, 122, 249–253. [Google Scholar]
- Wyss, M.; Kaddurah, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar]
- Demant, T.W.; Rhodes, D.E.C. Effects of Creatine Supplementation on Exercise Performance. Sports Med. 1999, 28, 49–60. [Google Scholar]
- Purchas, R.W.; Rutherfurd, S.M.; Pearce, P.D.; Vather, R.; Wilkinson, B.H.P. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q10, and creatine. Meat Sci. 2004, 66, 629–637. [Google Scholar]
- Kuchibamanabe, M.; Matoba, T.; Hasegawa, K. Sensory changes in umami taste of inosine 5’-monophosphate solution after heating. J. Food Sci. 2010, 56, 1429–1432. [Google Scholar]
- Meelis, T.; Kaja, T.; Mari Ann, T.R.; Lene, M.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agricult. Food Chem. 2006, 54, 7769–7777. [Google Scholar]
- Watanabe, A.; Tsuneishi, E.; Takimoto, Y. Analysis of ATP and Its Breakdown Products in Beef by Reversed-Phase HPLC. J. Food Sci. 2010, 54, 1169–1172. [Google Scholar]
- Ishiwatari, N.; Fukuoka, M.; Hamada-Sato, N.; Sakai, N. Decomposition kinetics of umami component during meat cooking. J. Food Eng. 2013, 119, 324–331. [Google Scholar]
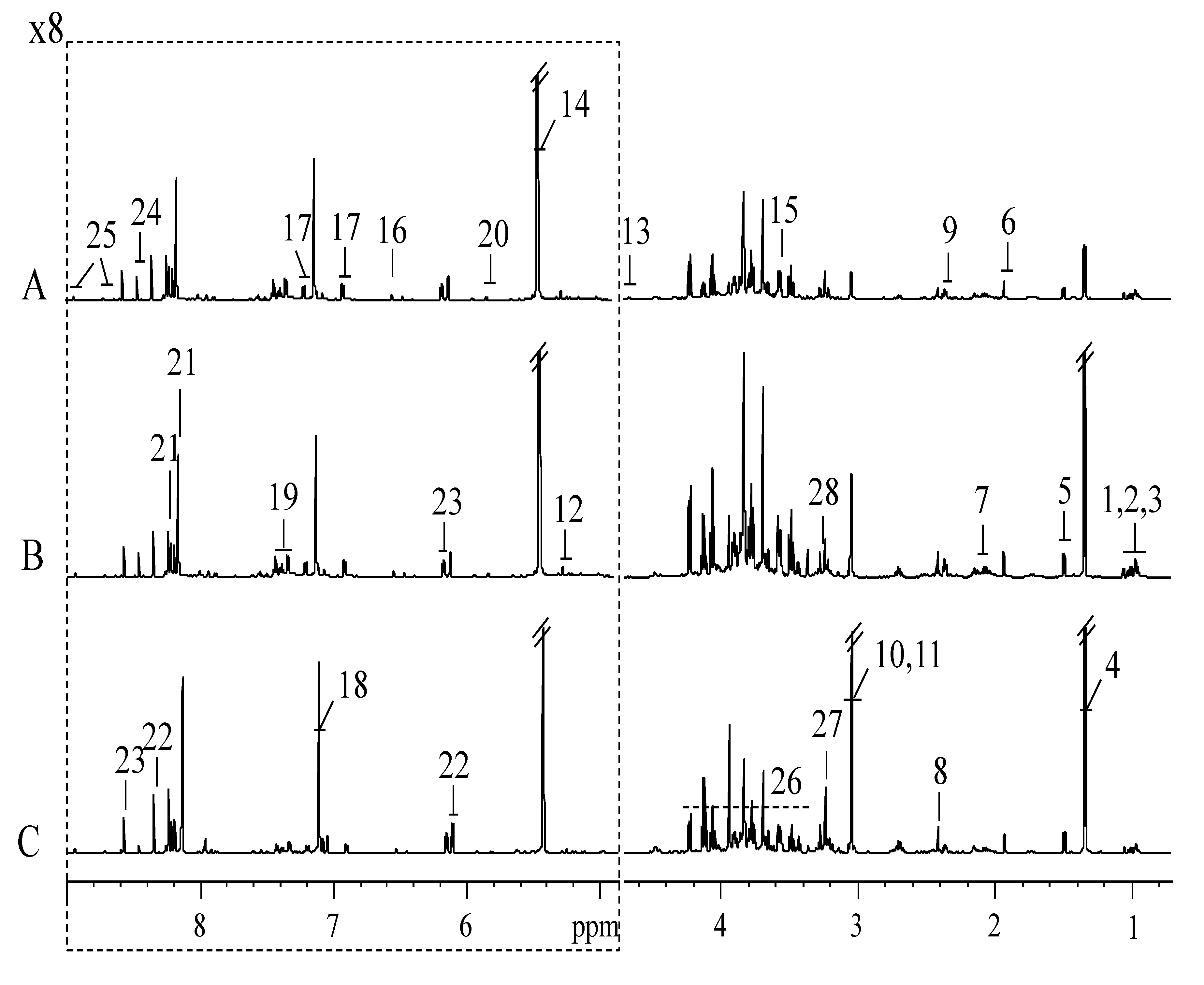
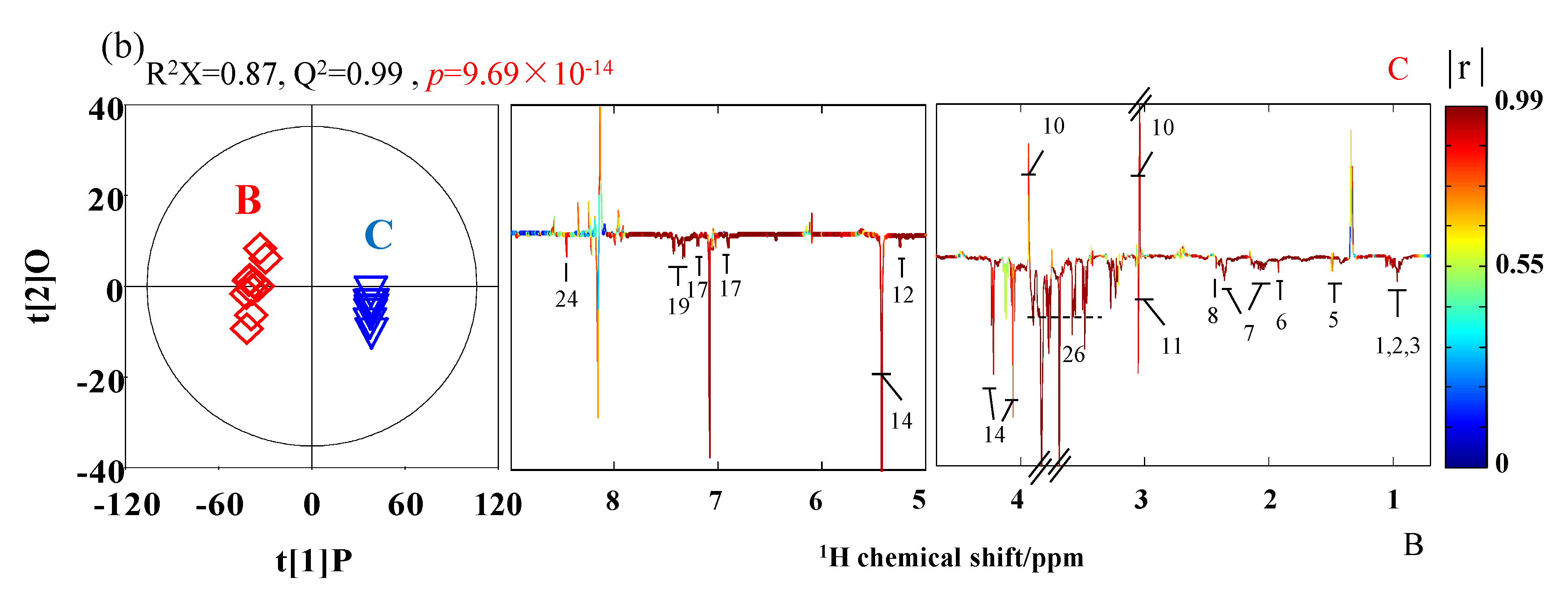
| Key | Metabolites | Moieties | δ1 H (ppm) and Multiplicitya | δ13 C (ppm) |
|---|---|---|---|---|
| 1 | Isoleucine | αCH, βCH, γCH2, γ’CH3 δCH3 | 3.67(d), 1.98(m), 1.26(m), 1.45(m), 1.01(d), 0.94(t) | 62.4, 38.6, 27.1, 17.7, 13.9 |
| 2 | Leucine | αCH, βCH2, γCH, δCH3, δ’CH3 | 3.73(m), 1.73(m), 1.67(m), 0.98(d), 0.96(d) | 56.3, 42.7, 26.8, 24.8, 23.8 |
| 3 | Valine | αCH, βCH, γCH3, γ’CH3 | 3.62(d), 2.27(m), 0.99(d), 1.04(d) | 63.2, 31.8, 19.3 |
| 4 | Lactate | αCH, βCH3, COOH | 4.11(q), 1.33(d) | 71.2, 23.1, 185.3 |
| 5 | Alanine | αCH, βCH3, COOH | 3.78(q), 1.48(d) | 53.3, 19.3, 178.7 |
| 6 | Acetate | CH3, COOH | 1.92(s) | 26.3, 184.3 |
| 7 | Glutamate | δCO, αCH, βCH2, γCH2, COOH | 3.77(m), 2.12(m), 2.05(m), 2.36(dt) | 184.2, 57.1, 29.8, 36.2, 177.7 |
| 8 | Succinate | CH2 | 2.41(s) | 36.9, 184.6 |
| 9 | Pyroglutamate | αCH, βCH2, γCH2 | 4.19(dd), 2.52(m), 2.04(m), 2.41(m), | 61.2, 28.3, 32.9 |
| 10 | Creatine | CH2, NCH3, CNH, COOH | 3.93(s), 3.04(s) | 39.8, 56.8, 160.3, 177.7 |
| 11 | Creatinine | CH2, NCH3, CNH, CO | 4.06(s), 3.05(s) | 59.1, 32.9, 172.1, 191.6 |
| 12 | α-Glucose | C1H, C2H, C3H | 5.24(d), 3.54(d) | 95.0, 73.0 |
| 13 | β-Glucose | C1H, C2H, C3H | 4.65(d), 3.25(t), 3.50(dd) | 98.6, 77.1, 78.7 |
| 14 | Sucrose | G1H, G2H, G3H, G4H, G5H, G6H, F1H, F2, F3H, F4H, F5H, F6H | 5.42(d), 3.58(dd), 3.77(t), 3.49(t), 3.82(q), 3.81(q), 3.69(s), 4.23(d), 4.06(t), 3.91(m), 3.83 | 94.7, 73.5, 75.0, 71.8, 74.9, 62.8, 64.0, 106.1, 79.2, 76.6, 83.7, 65.0 |
| 15 | Glycine | αCH2, COOH | 3.57(s) | 44.5, 175.4 |
| 16 | Fumarate | CH, COOH | 6.53(s) | 138.7, 177.5 |
| 17 | Tyrosine | C1, Ring, C2,6H, Ring, C3,5H, Ring, C4, Ring | 7.20(d), 6.90(d) | 129.5, 133.6, 118.8, 157.5 |
| 18 | Histidine | αCH, βCH, γC | 8.06(s), 7.12(d) | 136.8, 133.8, 119.7 |
| 19 | Phenylalanine | C1, Ring, C2,6H, Ring, C3,5H, Ring, C4H, Ring | 7.33(d), 7.43(t), 7.38(m) | 137.9, 132.6, 132.0, 130.7 |
| 20 | Uracil | C3H, C4H, C1, C2 | 7.54(d), 5.81(d) | 144.6, 103.4, 170.2, 156.1 |
| 21 | Hypoxanthine | C2H, C6H | 8.22(s), 8.20(s) | 144.6, 147.8 |
| 22 | Inosine | C1H, C2, C3, C4H, C5, C1′H, C2′H, C3′H, C4′H, C5′H | 8.34(s), 8.23(s), 6.10(d), 4.79, 4.44(m), 4.28 (m), 3.91, 3.83 | 143.0, 127.2, 161.8, 149.1, 151.5, 91.2, 77.1, 73.3, 88.4, 63.9 |
| 23 | 5′-IMP | C1H, C2, C3, C4H, C5, C1′H, C2′H, C3′H, C4′H, C5′H | 8.58(s), 8.24(s), 6.15(d), 4.81(#), 4.51(m), 4.38 (m), 4.06(#) | 142.7, 126.7, 161.8, 149.1, 152.0, 90.2, 77.6, 73.5, 87.4, 66.7 |
| 24 | Formate | CH | 8.46(s) | 174.2 |
| 25 | Nicotinamide | C2H, C4H, C5H, C6H | 8.94(dd), 8.27(dd), 7.60(dd), 8.72(dd) | 150.1, 139.0, 127.0, 154.2 |
| 26 | Sugars and amino acids | αCH resonances | 3.3-3.9 | |
| 27 | Phosphorylcholine | αCH2, βCH2, N-CH3 | 4.08(d), 3.55 (d), 3.23(s) | 69.0, 56.2 |
| 28 | Betaine | CH3, CH2, COOH | 3.28(s), 3.93(s) | 56.7, 68.9, 172.2 |
| Metabolite | Coefficient 1 | Mean ± SD 2 | |||
|---|---|---|---|---|---|
| B/A | C/B | A (mg/g) | B (mg/g) | C (mg/g) | |
| Isoleucine | 0.99 | −0.97 | 1.87 ± 0.02b | 2.95 ± 0.04a | 1.80 ± 0.09 b |
| Leucine | 0.99 | −0.99 | 0.19 ± 0.00b | 0.34 ± 0.02a | 0.16 ± 0.02c |
| Valine | 0.98 | −0.99 | 0.13 ± 0.00b | 0.26 ± 0.01a | 0.13 ± 0.01b |
| Alanine | 0.83 | −0.92 | 0.23 ± 0.00c | 0.50 ± 0.02a | 0.37 ± 0.04b |
| Glutamate | 0.99 | −0.99 | 3.19 ± 0.02a | 2.22 ± 0.10b | 1.01 ± 0.02c |
| Pyroglutamate | 0.99 | −0.99 | 0.87 ± 0.01b | 1.85 ± 0.08a | 0.88 ± 0.05b |
| Glycine | / | / | / | / | / |
| Tyrosine | 0.94 | −0.95 | 0.11 ± 0.00b | 0.18 ± 0.00a | 0.09 ± 0.01b |
| Histidine | −0.81 | −0.99 | 2.01 ± 0.27a | 1.83 ± 0.15b | 0.60 ± 0.01c |
| Phenylalanine | 0.97 | −0.99 | 0.17 ± 0.00b | 0.29 ± 0.01a | 0.13 ± 0.02c |
| Total FAAs | 8.77 | 10.42 | 5.17 | ||
| Lactate | 0.99 | 0.95 | 0.88 ± 0.01c | 3.81 ± 0.16b | 4.49 ± 0.51a |
| Acetate | 0.97 | −0.97 | 0.10 ± 0.00b | 0.17 ± 0.01a | 0.10 ± 0.01b |
| Succinate | 0.99 | −0.95 | 0.14 ± 0.00c | 0.37 ± 0.02a | 0.27 ± 0.03b |
| Creatine | 0.99 | 0.99 | 0.75 ± 0.10c | 1.93 ± 0.09b | 2.71 ± 0.31a |
| Fumarate | 0.91 | −0.86 | 0.01 ± 0.00b | 0.02 ± 0.00a | 0.01 ± 0.00b |
| Formate | − | − | 0.01 ± 0.00b | 0.04 ± 0.00a | 0.01 ± 0.00b |
| Total organic acids | 1.88 | 6.34 | 7.59 | ||
| α-Glucose | − | −0.98 | 0.19 ± 0.00a | 0.21 ± 0.03a | 0.07 ± 0.01b |
| β-Glucose | 0.96 | −0.99 | 0.31 ± 0.01b | 0.46 ± 0.03a | 0.15 ± 0.01c |
| Sucrose | 0.98 | −0.98 | 7.21 ± 0.10b | 15.81 ± 0.68a | 6.36 ± 0.74c |
| Total sugars | 7.71 | 16.48 | 6.58 | ||
| Uracil | − | − | 0.04 ± 0.00b | 0.07 ± 0.00a | 0.04 ± 0.00b |
| Hypoxanthine | 0.98 | −0.89 | 0.13 ± 0.00c | 0.27 ± 0.01a | 0.19 ± 0.02b |
| Inosine | 0.99 | − | 0.14 ± 0.00c | 0.51 ± 0.02b | 0.58 ± 0.07a |
| 5′-IMP | 0.99 | 0.87 | 0.05 ± 0.00c | 0.52 ± 0.02b | 0.59 ± 0.07a |
| Total nucleic acids and their derivatives | 0.36 | 1.37 | 1.4 | ||
| Creatinine | 0.81 | −0.92 | 1.14 ± 0.46b | 2.25 ± 0.43a | 1.03 ± 0.02c |
| Nicotinamide | 0.96 | − | 0.01 ± 0.00b | 0.05 ± 0.00a | 0.04 ± 0.00a |
| Phosphorylcholine | −0.87 | −0.98 | 0.96 ± 0.02a | 0.67 ± 0.02b | 0.34 ± 0.01c |
| Betaine | 0.99 | 0.99 | 0.67 ± 0.01c | 1.73 ± 0.08b | 2.51 ± 0.04a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Pan, D.; Wang, Y.; He, J.; Yue, Y.; Xia, Q.; Zhou, G.; Cao, J. Effect of Reconstituted Broth on the Taste-Active Metabolites and Sensory Quality of Stewed and Roasted Pork-Hock. Foods 2020, 9, 513. https://doi.org/10.3390/foods9040513
Yang Y, Pan D, Wang Y, He J, Yue Y, Xia Q, Zhou G, Cao J. Effect of Reconstituted Broth on the Taste-Active Metabolites and Sensory Quality of Stewed and Roasted Pork-Hock. Foods. 2020; 9(4):513. https://doi.org/10.3390/foods9040513
Chicago/Turabian StyleYang, Yi, Daodong Pan, Ying Wang, Jun He, Yi Yue, Qiang Xia, Guanghong Zhou, and Jinxuan Cao. 2020. "Effect of Reconstituted Broth on the Taste-Active Metabolites and Sensory Quality of Stewed and Roasted Pork-Hock" Foods 9, no. 4: 513. https://doi.org/10.3390/foods9040513
APA StyleYang, Y., Pan, D., Wang, Y., He, J., Yue, Y., Xia, Q., Zhou, G., & Cao, J. (2020). Effect of Reconstituted Broth on the Taste-Active Metabolites and Sensory Quality of Stewed and Roasted Pork-Hock. Foods, 9(4), 513. https://doi.org/10.3390/foods9040513