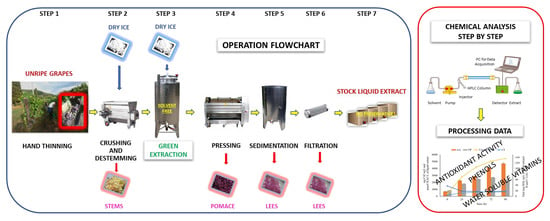
Recovery of Bioactive Compounds from Unripe Red Grapes (cv. Sangiovese) through a Green Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapes
2.2. Processing of Liquid Extracts
2.3. Chemicals
2.4. Grape Chemical Characteristics and Phenolic Maturity
2.5. Chemical Characterization of the Liquid Extracts
2.6. Total Phenol Content
2.7. Total Anthocyanin Content
2.8. Colour Intensity and Hue
2.9. Phenolic Composition and Glutathione Content
2.10. Water-Soluble vitamin Contents
2.11. Antioxidant Activity
2.12. Data Processing
3. Results and Discussion
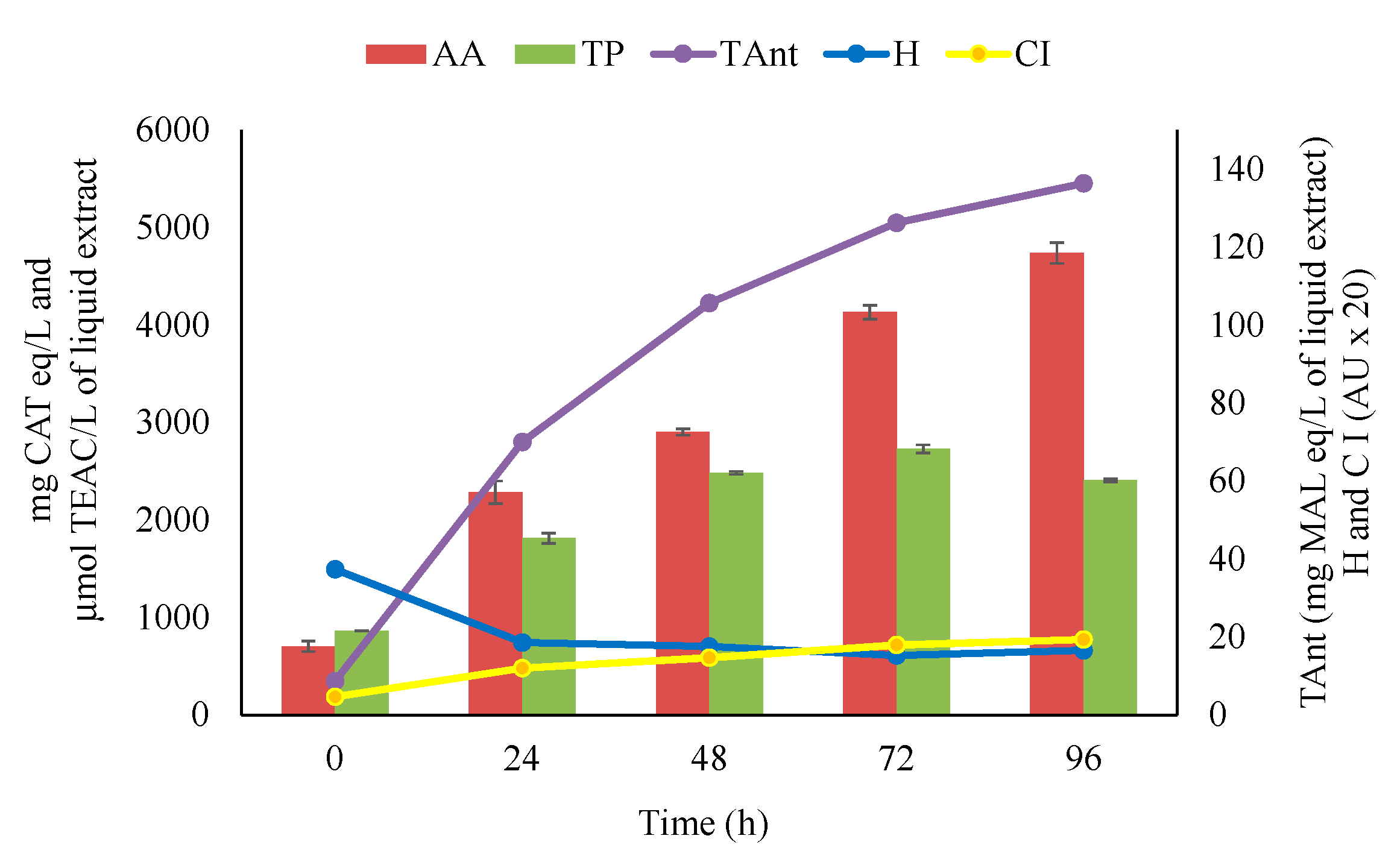
3.1. Recovery of Bioactive Compounds during Extraction by Maceration
3.2. Recovery of Bioactive Compounds during Post-Extraction Steps
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT-Food Sci. Technol. 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Hogan, S.; Zhang, L.; Li, J.; Zoecklein, B.; Zhou, K. Antioxidant properties and bioactive components of Norton (Vitis aestivalis) and Cabernet Franc (Vitis vinifera) wine grapes. LWT-Food Sci. Technol. 2009, 42, 1269–1274. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Gratacós-Cubarsí, M.; Sárraga, C.; Guàrdia, M.D.; García-Regueiro, J.A.; Castellari, M. Stability of phenolic compounds in dry fermented sausages added with cocoa and grape seed extracts. LWT-Food Sci. Technol. 2014, 57, 329–336. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. LWT-Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Gatti, M.; Bernizzoni, F.; Civardi, S.; Poni, S. Effects of cluster thinning and preflowering leaf removal on growth and grape composition in cv. Sangiovese. Am. J. Enol. Vitic. 2012, 63, 325–332. [Google Scholar] [CrossRef]
- Ough, C.S.; Nagaoka, R. Effect of Cluster Thinning and Vineyard Yields on Grape and Wine Composition and Wine Quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1984, 35, 30–34. [Google Scholar]
- Öncül, N.; Karabiyikli, Ş. Factors Affecting the Quality Attributes of Unripe Grape Functional Food Products. J. Food Biochem. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Evaluation of antibrowning and antioxidant activities in unripe grapes recovered during bunch thinning. Aust. J. Grape Wine Res. 2016, 23, 33–41. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of Dipping Pre-treatment with Unripe Grape Juice on Dried “Golden Delicious” Apple Slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- De Dupas Matos, A.; Curioni, A.; Bakalinsky, A.T.; Marangon, M.; Pasini, G.; Vincenzi, S. Chemical and sensory analysis of verjuice: An acidic food ingredient obtained from unripe grape berries. Innov. Food Sci. Emerg. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- De Dupas Matos, A.; Magli, M.; Marangon, M.; Curioni, A.; Pasini, G.; Vincenzi, S. Use of verjuice as an acidic salad seasoning ingredient: Evaluation by consumers’ liking and Check-All-That-Apply. Eur. Food Res. Technol. 2018, 244, 2117–2125. [Google Scholar] [CrossRef]
- De Dupas Matos, A.; Marangon, M.; Magli, M.; Cianciabella, M.; Predieri, S.; Curioni, A.; Vincenzi, S. Sensory characterization of cucumbers pickled with verjuice as novel acidifying agent. Food Chem. 2019, 286, 78–86. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Keramat, M.; Moosavi-Nasab, M.; Moosavian, B. Oxidative Stability of Common Kilka (Clupeonella cultriventris caspia) Oil Supplemented with Microwave Extracted Ghure (Unripe Grape) Marc Extract. J. Aquat. Food Prod. Technol. 2017, 26, 1022–1031. [Google Scholar] [CrossRef]
- Öncül, N.; Karabıyıklı, Ş. Survival of foodborne pathogens in unripe grape products. LWT-Food Sci. Technol. 2016, 74, 168–175. [Google Scholar] [CrossRef]
- Turkmen, F.U.; Takci, H.A.M.; Sekeroglu, N. Total phenolic and flavonoid contents, antioxidant and antimicrobial activities of traditional unripe grape products. Indian J. Pharm. Educ. Res. 2017, 51, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Renard, C.M.G.C. Extraction of bioactives from fruit and vegetables: State of the art and perspectives. LWT-Food Sci. Technol. 2018, 93, 390–395. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, S.M.; Tiwari, B.; Hashemi, M.; Fadavi, G.; Khaksar, R. Polyphenols content and antioxidant activity of Ghure (unripe grape) marc extract: Influence of extraction time, temperature and solvent type. Int. J. Food Sci. Technol. 2013, 48, 412–418. [Google Scholar] [CrossRef]
- Fia, G.; Gori, C.; Bucalossi, G.; Borghini, F.; Zanoni, B. A Naturally Occurring Antioxidant Complex from Unripe Grapes: The Case of Sangiovese (v. Vitis vinifera). Antioxidants 2018, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Toschi, T.G.; Monteleone, E. Functional and sensory proprieties of phenolic compounds from unripe grapes in vegetable food prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches, A.S.; Vilarinho, F.; Casilho, C.M.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Gori, C.; Menichetti, S.; Fia, G. Multi-functional oenological machine and use in the oenological production chain. European Patent Number 2,957,627, 22 June 2014. [Google Scholar]
- Fia, G.; Gori, C. Process for the extraction of antioxidants from plant matrices. Italian Patent Number 102,016,000,022,015, 2 March 2016. [Google Scholar]
- De Saint-Cricq Gaulejac, N.; Vivas, N.; Glories, Y. Maturité phénolique: Définition et contrôle. Rev. Fr. D’oenologie 1998, 173, 22–25. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Rigo, A.; Vianello, F.; Clementi, G.; Rossetto, M.; Scarpa, M.; Vrohvšek, U.; Mattivi, F. Contribution of proanthocyanidins to the peroxy radical scavenging capacity of some Italian red wines. J. Agric. Food Chem. 2000, 48, 1996–2002. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. lre partie: Les équilibres des anthocyanes et des tanins. OENO One 1984, 18, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Owen, S.C.; Lee, M.; Grissom, C.B. Ultra-performance liquid chromatographic separation and mass- spectrometric quantitation of physiologic cobalamins. J. Chromatogr. Sci. 2011, 49, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Canuti, V.; Picchi, M.; Zanoni, B.; Fia, G.; Bertuccioli, M. A multivariate methodological approach to relate wine to characteristics of grape composition: The case of typicality. Am. J. Enol. Vitic. 2017, 68, 49–59. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; duToit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT-Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Calamita, Y.; Andrich, G. L’impiego della criomacerazione nella vinificazione di uve Sangiovese. Boll. Della Comunità Sci. Australas. 2008, 12, 47–56. [Google Scholar]
- Zanoni, B.; Siliani, S.; Canuti, V.; Rosi, I.; Bertuccioli, M. A kinetic study on extraction and transformation phenomena of phenolic compounds during red wine fermentation. Int. J. Food Sci. Technol. 2010, 45, 2080–2088. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grapes: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Breksa, A.P., III; Takeoka, G.R.; Hidalgo, M.B.; Vilches, A.; Vasse, J.; Ramming, D.W. Antioxidant activity and phenolic content of 16 raisin grape (Vitis vinifera L.) cultivars and selections. Food Chem. 2010, 121, 740–745. [Google Scholar] [CrossRef]
- Orak, H.H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hortic. 2007, 111, 235–241. [Google Scholar] [CrossRef]
- Bartolomé, B.; Nuñez, V.; Monagas, M.; Gómez-Cordovés, C. In vitro antioxidant activity of red grape skins. Eur. Food Res. Technol. 2004, 218, 173–177. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Hagen, K.M.; Keller, M.; Edwards, C.G. Survey of Biotin, Pantothenic Acid, and Assimilable Nitrogen in Winegrapes from the Pacific Northwest. Am. J. Enol. Vitic. 2008, 59, 432–436. [Google Scholar]
- Motta, S.; Guaita, M.; Petrozziello, M.; Panero, L.; Bosso, A. Effect of Reductive Pressing on the Concentration of Reduced Glutathione and Phenols in the Musts of Four Italian Cultivars. Am. J. Enol. Vitic. 2014, 65, 471–478. [Google Scholar] [CrossRef]

| S | pH | TA | P/J Ratio * | ApH1.0 | ApH3.2 | EA% | A280 | Mp% | dTskin | dTseed |
|---|---|---|---|---|---|---|---|---|---|---|
| 143.0 ± 1.5 | 3.3 ± 0.02 | 5.7 ± 0.1 | 112.2 ± 4.0 | 1035.8 ± 30 | 513.5 ± 15 | 50.4 ± 1.6 | 23.1 ± 1.1 | 11.1 ± 0.8 | 20.5 ± 1.4 | 2.6 ± 0.03 |
| Variables | Time (h) | ||||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | |
| TP | 864 d | 1813 c | 2483 b | 2728 a | 2407 b |
| AA | 702 d | 2282 c | 2902 b | 4134 a | 4738 a |
| CI | 0.2 e | 0.6 d | 0.7 c | 0.9 b | 1.0 a |
| H | 1.9 a | 0.9 b | 0.9 b | 0.8 c | 0.8 c |
| TAnt | 8.7 d | 70.0 c | 105.6 b | 126.3 a | 136.3 a |
| Compound | Extraction | After Filtration | ||
|---|---|---|---|---|
| 0 h | 48 h | 96 h | ||
| Content (mg/L) | ||||
| Phenolic acids | ||||
| Caffeic acid | nd | nd | nd | nd |
| Caftaric acid | 31.63 ± 1.98 d | 59.00 ± 2.89 c | 86.65 ± 4.49 a | 135.3 ± 3.36 a |
| Coumaric acid | nd | nd | nd | nd |
| Coutaric acid | 0.30 ± 0.05 d | 10.19 ± 0.18 c | 36.62 ± 0.41 b | 74.41 ± 3.49 b |
| Ferulic acid | nd | nd | nd | nd |
| Fertaric acid | 0.09 ± 0.01 d | 2.85 ± 0.07 c | 8.41 ± 0.06 b | 33.06 ± 0.96 a |
| Gallic acid | nd | nd | nd | 0.06 ± 0.00 |
| Flavonols | ||||
| Quercetin | 0.06 ± 0.06 a | 0.05 ± 0.00 a | 0.06 ± 0.00 a | 0.05 ± 0.00 a |
| Quercetin 3-O-hexoside | 0.72 ± 0.18 c | 4.89 ± 0.37 b | 12.86 ± 0.09 a | 12.33 ± 0.46 b |
| Quercetin 3-O-glucuronide | 1.59 ± 0.20 d | 8.99 ± 0.75 c | 32.51 ± 0.60 a | 23.54 ± 0.69 b |
| Rutin | 0.17 ± 0.04 c | 0.61 ± 0.05 b | 0.83 ± 0.03 a | 0.86 ± 0.03 a |
| Isorhamnetin | nd | nd | nd | nd |
| Kaempferol | nd | nd | nd | nd |
| Kaempferol 3-O-glucoside | 0.34 ± 0.01 c | 1.03 ± 0.04 b | 2.64 ± 0.05 a | 2.43 ± 0.14 a |
| Myricetin | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.04 ± 0.02 a |
| Myricetin-hexoside | nd | nd | nd | nd |
| Piceatannol | nd | nd | nd | nd |
| Flavan-3-ols | ||||
| (+)-Catechin | 0.10 ± 0.03 d | 17.96 ± 0.12 c | 80.20 ± 1.18 b | 156.31±1.29 a |
| (−)-Epicatechin | nd | 0.49 ± 0.09 c | 7.35 ± 0.18 b | 17.21 ± 0.42 a |
| Epicatechin-O-gallate | nd | nd | 0.12 ± 0.01 a | 0.15 ± 0.01 a |
| Procyanidins | ||||
| Procyanidin B1 | nd | 3.04 ± 0.05 c | 9.22 ± 0.08 b | 10.30 ± 0.15 a |
| Procyanidin B2 | 0.19 ± 0.05 d | 6.79 ± 0.05 c | 9.22 ± 0.08 b | 17.71 ± 0.15 a |
| Glutathione | ||||
| GSH | nd | nd | nd | nd |
| Grape reaction product-GRP | 1.97 ± 0.16 c | 6.23 ± 0.81 b | 7.48 ± 0.92 a | 6.39 ± 0.75 b |
| GSSG | 1.08 ± 0.06 ab | 1.03 ± 0.05 b | 1.18 ± 0.08 a | 1.14 ± 0.09 ab |
| Water-Soluble Vitamins | Extraction | |||
|---|---|---|---|---|
| 0 h | 48 h | 96 h | After Filtration | |
| Content (µg/L) | ||||
| Folic acid (B9) | nd | nd | nd | nd |
| Pantothenic acid (B5) | 360.3 ± 10.4 b | 455.7 ± 24.8 ab | 492.0 ± 77.7 a | 452.0 ± 82.5 ab |
| Ascorbic acid (C) | nd | nd | nd | nd |
| Biotin (B7) | nd | nd | nd | nd |
| Cobalamin (B12) | nd | nd | nd | nd |
| Choline (J) | 400.3 ± 27.0 c | 603.3 ± 62.3 b | 779.7 ± 148.9 a | 782.3 ± 29.3 a |
| Niacin (B3) | 53.7 ± 5.9 b | 58.7 ± 5.5 ab | 51.0 ± 7.0 b | 62.0 ± 2.6 a |
| Pyridoxal (B6) | nd | nd | nd | nd |
| Pyridoxamine (B6) | nd | nd | nd | nd |
| Pyridoxine (B6) | 93.0 ± 12.1 a | 94.0 ± 7.0 a | 110.0 ± 7.9 a | 100.7 ± 8.4 a |
| Riboflavin (B2) | nd | nd | nd | nd |
| Thiamine (B1) | nd | nd | nd | nd |
| Total content | 906 | 1212 | 1433 | 1397 |
| Parameter | End of Extraction | Post-Pressing | Post-Decantation | Post-Filtration |
|---|---|---|---|---|
| TP | 2407 ± 30 b | 2793 ± 70 a | 2827 ± 30 a | 2522 ± 40 b |
| AA | 4738 ± 107 b | 8103 ± 793 a | 8130 ± 225 a | 8277 ± 731 a |
| CI | 0.97 ± 0.0 a | 0.91 ± 0.0 b | 0.89 ± 0.0 c | 0.77 ± 0.0 d |
| H | 0.83 ± 0.0 a | 0.75 ± 0.0 b | 0.83 ± 0.0 a | 0.73 ± 0.0 c |
| TAnt | 136.3 ± 10 a | 142.3 ± 16 a | 129.4 ± 4.0 a | 132.9 ± 4.0 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fia, G.; Bucalossi, G.; Gori, C.; Borghini, F.; Zanoni, B. Recovery of Bioactive Compounds from Unripe Red Grapes (cv. Sangiovese) through a Green Extraction. Foods 2020, 9, 566. https://doi.org/10.3390/foods9050566
Fia G, Bucalossi G, Gori C, Borghini F, Zanoni B. Recovery of Bioactive Compounds from Unripe Red Grapes (cv. Sangiovese) through a Green Extraction. Foods. 2020; 9(5):566. https://doi.org/10.3390/foods9050566
Chicago/Turabian StyleFia, Giovanna, Ginevra Bucalossi, Claudio Gori, Francesca Borghini, and Bruno Zanoni. 2020. "Recovery of Bioactive Compounds from Unripe Red Grapes (cv. Sangiovese) through a Green Extraction" Foods 9, no. 5: 566. https://doi.org/10.3390/foods9050566
APA StyleFia, G., Bucalossi, G., Gori, C., Borghini, F., & Zanoni, B. (2020). Recovery of Bioactive Compounds from Unripe Red Grapes (cv. Sangiovese) through a Green Extraction. Foods, 9(5), 566. https://doi.org/10.3390/foods9050566