Assessment of the Miniature Kramer Shear Cell to Measure Both Solid Food and Bolus Mechanical Properties and Their Interplay with Oral Processing Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid Food Samples
2.2. Methods
2.2.1. Physical Measurements of Solid Foods
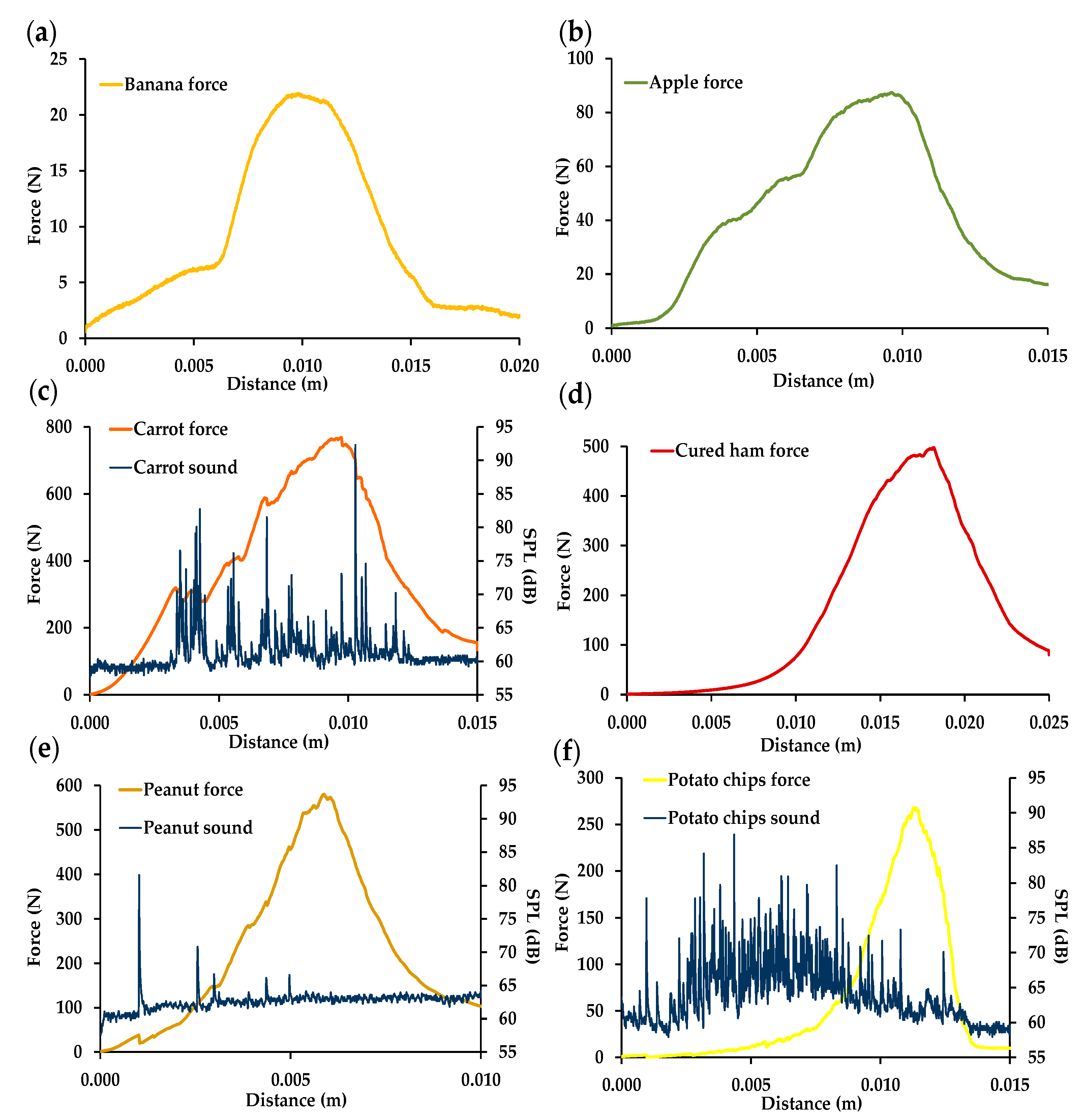
Moisture Determination
Kramer Mechanical Properties
Acoustic Properties
2.2.2. Physical Measurements of Bolus Counterparts
Kramer Mechanical Properties
Cone Penetration (CP) Mechanical Properties
Small Amplitude Oscillatory Strain (SAOS) Rheological Properties
Particle Characterization by Granulometric Analysis
2.2.3. Bolus Formation and Oral Processing Characteristics
2.3. Data Analysis
3. Results and Discussion
3.1. Instrumental Measurements
3.1.1. Kramer Mechanical and Acoustic Properties of Foods
3.1.2. Mechanical Properties of Boluses
3.1.3. Particle Size Characterization of Boluses
3.1.4. Rheological Properties of Boluses
3.2. Oral Processing Behaviors
3.2.1. Obtained from a Healthy Young Male Volunteer
3.2.2. Obtained from 39 Healthy Untrained Volunteers
3.3. Relation between Measured Physical Properties and Oral Processing Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Hutchings, J.B.; Lillford, P.J. The perception of food texture—The philosophy of the breakdown path. J. Texture Stud. 1988, 19, 103–115. [Google Scholar] [CrossRef]
- Van Vliet, T. On the relation between texture perception and fundamental mechanical parameters for liquids and time dependent solids. Food Qual. Prefer. 2002, 13, 227–236. [Google Scholar] [CrossRef]
- Bourne, M.C. Principles of objective texture measurement. In Food Texture and Viscosity (Concept and Measurement); Bourne, M.C., Ed.; Academic Press: London, UK, 2002; pp. 107–187. [Google Scholar]
- Koç, H.; Çakir, E.; Vinyard, C.J.; Essick, G.; Dauberti, C.R.; Drake, M.A.; Osborne, J.; Foegeding, E.A. Adaptation of oral processing to the fracture properties of soft solids. J. Texture Stud. 2014, 45, 47–61. [Google Scholar] [CrossRef]
- Devezeaux de Lavergne, M.; Tournier, C.; Bertrand, D.; Salles, C.; van de Velde, F.; Stieger, M. Dynamic texture perception, oral processing behaviour and bolus properties of emulsion-filled gels with and without contrasting mechanical properties. Food Hydrocoll. 2016, 52, 648–660. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Perception and measurement of food texture: Solid foods. J. Texture Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human oral processing and texture profile analysis parameters: Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Rizo, A.; Peña, E.; Alarcon-Rojo, A.D.; Fiszman, S.; Tarrega, A. Relating texture perception of cooked ham to the bolus evolution in the mouth. Food Res. Int. 2019, 118, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef]
- Laguna, L.; Barrowclough, R.A.; Chen, J.; Sarkar, A. New approach to food difficulty perception: Food structure, food oral processing and individual’s physical strength. J. Texture Stud. 2019, 47, 413–422. [Google Scholar] [CrossRef]
- Engelen, L.; Fontijn-Tekamp, A.; van der Bilt, A. The influence of product and oral characteristics on swallowing. Arch. Oral Biol. 2005, 50, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Krop, E.M.; Hetherington, M.M.; Miquel, S.; Sarkar, A. Oral processing of hydrogels: Influence of food material properties versus individuals’ eating capability. J. Texture Stud. 2019, 51, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Objective measurements of food texture. J. Food Sci. 1963, 28, 410–420. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). International Standard 5492. In Sensory Analysis—Vocabulary; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- Alvarez, M.D.; Velarde, C.; Barrios, L.; Herranz, B. Understanding the crispy–crunchy texture of raw red pepper and its change with storage time. J. Texture Stud. 2019. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C.A. Influence of the starch content in the viscoelastic properties of surimi gels. J. Food Eng. 2008, 84, 140–147. [Google Scholar] [CrossRef]
- Peyron, M.A.; Santé-Lhoutellier, V.; François, O.; Hennequin, M. Oral declines and mastication deficiencies cause alteration of food bolus properties. Food Funct. 2018, 9, 1112–1122. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). International Standard 8589. In Sensory Analysis—General Guidance for the Design of Test Rooms; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Rojo, F.J.; Vincent, J.F.V. Fracture properties of potato crisps. Int. J. Food Sci. Technol. 2008, 43, 752–760. [Google Scholar] [CrossRef]
- Salvador, A.; Varela, P.; Sanz, T.; Fiszman, S.M. Understanding potato chips crispy texture by simultaneous fracture and acoustic measurements, and sensory analysis. LWT Food Sci. Technol. 2009, 42, 763–767. [Google Scholar] [CrossRef]
- Fiszman, S.; Tarrega, A. The dynamics of texture perception of hard solid food: A review of the contribution of the temporal dominance of sensations technique. J. Texture Stud. 2018, 49, 202–212. [Google Scholar] [CrossRef]
- Hiiemae, K.; Heath, M.R.; Heath, G.; Kazazoglu, E.; Murray, J.; Sapper, D.; Hamblett, K. Natural bites, food consistency and feeding behaviour in man. Arch. Oral Biol. 1996, 41, 175–189. [Google Scholar] [CrossRef]
- Devezeaux de Lavergne, M.; van de Velde, F.; Stieger, M. Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food Funct. 2017, 8, 464–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Kim, D.-K.; Bae, J.H.; Kang, S.H.; Seo, K.M.; Kim, B.K.; Lee, S.Y. The effect of rheological properties of foods on bolus characteristics after mastication. Ann. Rehabil. Med. 2012, 36, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, A.E.; Veyrune, J.L.; Nicolas, E.; Ciangura, C.A.; Chaussain, C.C.; Czernichow, S.; Basdevant, A.; Hennequin, M. Effect of dental status on changes in mastication in patients with obesity following bariatric surgery. PLoS ONE 2011, 6, e22324. [Google Scholar] [CrossRef] [PubMed]
- Woda, A.; Nicolas, E.; Mishellany-Dutour, A.; Hennequin, M.; Mazille, M.N.; Veyrune, J.L.; Peyron, M.A. The masticatory normative indicator. J. Dent. Res. 2010, 89, 281–285. [Google Scholar] [CrossRef]
- Peyron, M.A.; Mishellany, A.; Woda, A. Particle size distribution of food boluses after mastication of six natural foods. J. Dent. Res. 2004, 83, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Jalabert-Malbos, M.L.; Mishellany-Dutour, A.; Woda, A.; Peyron, M.A. Particle size distribution in the food bolus after mastication of natural foods. Food Qual. Prefer. 2007, 18, 803–812. [Google Scholar] [CrossRef]
- Chen, J.; Khandelwal, N.; Liu, Z.; Funami, T. Influences of food hardness on the particle size distribution of food boluses. Arch. Oral Biol. 2013, 58, 293–298. [Google Scholar] [CrossRef]
- Bonnet, G.; Batisse, C.; Peyron, M.A.; Nicolas, E.; Hennequin, M. Which variables should be controlled when measuring the granulometry of a chewed bolus? A systematic review. J. Texture Stud. 2019, 50, 194–216. [Google Scholar] [CrossRef]
- Solo-de-Zaldívar, B.; Tovar, C.A.; Borderías, A.J.; Herranz, B. Pasteurization and chilled storage of restructured fish muscle products based on glucomannan gelation. Food Hydrocoll. 2015, 43, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Herranz, B.; Tovar, C.A.; Solo-de-Zaldívar, B.; Borderias, A.J. Effect of alkalis on konjac glucomannan gels for use as potential gelling agents in restructured seafood products. Food Hydrocoll. 2012, 27, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Sukkar, S.G.; Maggi, N.; Travalca Cupillo, B.; Ruggiero, C. Optimizing texture modified foods for oro-pharyngeal dysphagia: A difficult but possible target? Front. Nutr. 2018, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, C.G.; Leong, C.; Chia-Ming, E.; McCrickerd, K. Fast or slow-foods? Describing natural variations in oral processing characteristics across a wide range of Asian foods. Food Funct. 2017, 8, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar]
- Gavião, M.B.D.; Engelen, L.; van der Bilt, A. Chewing behavior and salivary secretion. Eur. J. Oral Sci. 2004, 112, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, A.M.; Hernández-Sánchez, F. Masticatory performance parameters for young adults with “normal” occlusion. CRANIO 2019, 37, 317–322. [Google Scholar] [CrossRef]
- Claveria, O.; Poluzzi, A. Positioning and clustering of the world’s top tourist destinations by means of dimensionality reduction techniques for categorical data. J. Destin. Mark. Manag. 2017, 6, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [Green Version]


| Food | Food Weight (g) | FKMF (N) | FKAF (N) | FKW (J) | Force Peaks | SPLmax (dB) | Sound Peaks | Average Drop-off | FMC (%) |
|---|---|---|---|---|---|---|---|---|---|
| Banana | 6.44a (0.134) | 23.4d (1.53) | 8.08c (0.591) | 0.195e (0.017) | - | - | - | - | 81.4b (2.91) |
| Apple | 5.28 b (0.217) | 83.8d (4.87) | 31.1c (0.450) | 0.779d (0.011) | - | - | - | - | 89.1a (0.324) |
| Carrot | 6.42a (0.390) | 725a (42.3) | 377 a (24.5) | 5.65a (0.368) | 25.0a (3.58) | 90.0a (5.50) | 24.0a (3.65) | 7.94a (0.814) | 92.2a (0.009) |
| Cured Ham | 6.30a (0.141) | 554b (71.4) | 201b (21.0) | 5.03b (0.525) | - | - | - | - | 58.1c (1.95) |
| Peanut | 5.52b (0.130) | 574b (16.0) | 200b (23.8) | 2.48c (0.310) | 13.0b (1.79) | 80.6b (3.97) | 12.0b (1.67) | 6.11b (0.823) | 4.65d (1.17) |
| Potato Chips | 1.24c (0.182) | 244c (0.091) | 36.8c (3.63) | 0.921d (0.091) | 26.0a (3.00) | 86.9a,b (1.75) | 25.0a (3.36) | 7.25a,b (0.450) | 5.12d (0.352) |
| Food | BKMF (N) | BKAF (N) | BKW (J) | BCPC (N) | BCPC Per Gram (N g−1) | BCPAF (N) | BCPW (J) |
|---|---|---|---|---|---|---|---|
| Banana | 4.84 d | 1.79d | 0.036 c | 5.56 e | 0.823 e | 0.937e | 0.006 c |
| (0.528) | (0.092) | (0.002) | (0.534) | (0.118) | (0.080) | (0.000) | |
| Apple | 8.07 d | 3.35 c,d | 0.050 c | 68.2 d | 11.4 d | 5.33 d | 0.045 c |
| (0.972) | (0.489) | (0.007) | (3.88) | (0.646) | (0.582) | (0.003) | |
| Carrot | 197 b | 63.3 b | 1.58 b | 478 a | 82.9 a | 33.2 a | 0.341 a |
| (16.8) | (3.42) | (0.086) | (29.3) | (8.57) | (3.14) | (0.042) | |
| Cured Ham | 251 a | 84.6 a | 1.86 a | 265 b | 38.9 b | 27.0 b | 0.360 a |
| (31.1) | (13.1) | (0.288) | (30.1) | (3.22) | (1.97) | (0.039) | |
| Peanut | 38.1 c | 12.8 c | 0.194 c | 135 c | 21.3 c | 10.8c | 0.133 b |
| (4.79) | (0.821) | (0.015) | (17.0) | (3.83) | (0.706) | (0.006) | |
| Potato Chips | 17.0 c,d | 2.85 c,d | 0.029 c | 24.1 e | 11.6 d | 3.41 d,e | 0.042 c |
| (0.972) | (0.311) | (0.003) | (2.26) | (1.42) | (0.213) | (0.003) |
| Food | σmax (kPa) | γmax (%) | G*max (kPa) | tan δ |
|---|---|---|---|---|
| Banana | 0.006 e | 0.251 c | 2.44 c | 0.285 a,b |
| (0.001) | (0.001) | (0.548) | (0.016) | |
| Apple | 0.029 e | 0.159 d | 18.2 c | 0.177 d |
| (0.006) | (0.001) | (3.58) | (0.010) | |
| Carrot | 0.222 a | 0.100 e | 222 a | 0.197 c,d |
| (0.008) | (0.001) | (9.45) | (0.005) | |
| Cured Ham | 0.135 c | 1.00 a | 13.4 c | 0.292 a |
| (0.005) | (0.001) | (0.550) | (0.023) | |
| Peanut | 0.175 b | 0.158 d | 111 b | 0.216 c |
| (0.021) | (0.000) | (12.9) | (0.002) | |
| Potato Chips | 0.080 d | 0.395 b | 20.2 c | 0.255 b |
| (0.010) | (0.000) | (2.45) | (0.004) |
| Food | Chewing Time (s) | Number of Chews | Chewing Rate (chews s−1) | Eating Rate (g min−1) | Food Weight (g) | Bolus Weight (g) | Saliva Uptake (SU) (g) |
|---|---|---|---|---|---|---|---|
| Banana | 9.20 d | 13 d | 1.42 b | 47.8 a | 7.29 a | 7.88 b | 0.588 c,d |
| (0.837) | (1.00) | (0.136) | (4.18) | (0.133) | (0.456) | (0.493) | |
| Apple | 9.40 d | 15 d | 1.59 a,b | 40.6 b | 6.29 b | 6.41 c | 0.120 d |
| (1.34) | (1.48) | (0.106) | (4.66) | (0.442) | (0.797) | (0.016) | |
| Carrot | 31.2 a | 45 b | 1.44 b | 14.3 c | 7.42 a | 9.02 a | 1.60 b |
| (1.48) | (1.30) | (0.052) | (0.979) | (0.213) | (0.316) | (0.124) | |
| Cured Ham | 27.8 b | 48 a | 1.74 a | 13.5 c | 6.26 b | 9.29 a | 3.03 a |
| (1.10) | (3.05) | (0.173) | (0.498) | (0.119) | (0.382) | (0.390) | |
| Peanut | 20.4 c | 28 c | 1.38 b | 16.1 c | 5.47 c | 7.00 b,c | 1.53 b |
| (1.14) | (1.79) | (0.096) | (0.763) | (0.202) | (0.887) | (0.716) | |
| Potato Chips | 8.60 d | 13 d | 1.46 b | 12.6 c | 1.80 d | 3.13 d | 1.33 b,c |
| (0.548) | (1.14) | (0.068) | (0.442) | (0.136) | (0.153) | (0.214) |
| Food | Chewing Time (s) | Number of Chews | Chewing Rate (chews s−1) | Eating Rate (g min−1) | Food Weight (g) | Bolus Weight (g) | Saliva Uptake (SU) (g) |
|---|---|---|---|---|---|---|---|
| Banana | 10.0 b | 11 b | 1.16 a | 43.8 a | 6.90 b | 6.78 a | −0.121 b,c |
| (3.76) | (4.70) | (0.319) | (14.0) | (1.96) | (1.84) | (1.40) | |
| Apple | 10.6 b | 14 b | 1.34 a | 49.9 a | 8.19 a | 7.74 a | −0.479 c |
| (4.06) | (5.25) | (0.263) | (18.2) | (2.69) | (2.97) | (1.73) | |
| Carrot | 19.8 a | 25 a | 1.29 a | 14.2 b | 4.48 c | 4.99 b | 0.518 a,b |
| (6.87) | (10.6) | (0.313) | (4.81) | (1.68) | (2.01) | (1.58) | |
| Cured Ham | 19.3 a | 25 a | 1.29 a | 8.50 b | 2.36 d | 3.58 c | 1.22 a |
| (8.06) | (10.5) | (0.280) | (3.80) | (0.562) | (1.04) | (0.723) | |
| Peanut | 10.1 b | 12 b | 1.30 a | 10.0 b | 1.53 d | 2.29 d | 0.759 a |
| (3.43) | (3.94) | (0.398) | (3.82) | (0.394) | (0.850) | (0.882) | |
| Potato Chips | 11.0 b | 13 b | 1.27 a | 11.4 b | 1.87 d | 3.15 c,d | 1.28 a |
| (3.97) | (4.33) | (0.348) | (4.82) | (0.436) | (1.11) | (1.00) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.D.; Paniagua, J.; Herranz, B. Assessment of the Miniature Kramer Shear Cell to Measure Both Solid Food and Bolus Mechanical Properties and Their Interplay with Oral Processing Behavior. Foods 2020, 9, 613. https://doi.org/10.3390/foods9050613
Álvarez MD, Paniagua J, Herranz B. Assessment of the Miniature Kramer Shear Cell to Measure Both Solid Food and Bolus Mechanical Properties and Their Interplay with Oral Processing Behavior. Foods. 2020; 9(5):613. https://doi.org/10.3390/foods9050613
Chicago/Turabian StyleÁlvarez, María Dolores, Jaime Paniagua, and Beatriz Herranz. 2020. "Assessment of the Miniature Kramer Shear Cell to Measure Both Solid Food and Bolus Mechanical Properties and Their Interplay with Oral Processing Behavior" Foods 9, no. 5: 613. https://doi.org/10.3390/foods9050613






