Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Cultivation
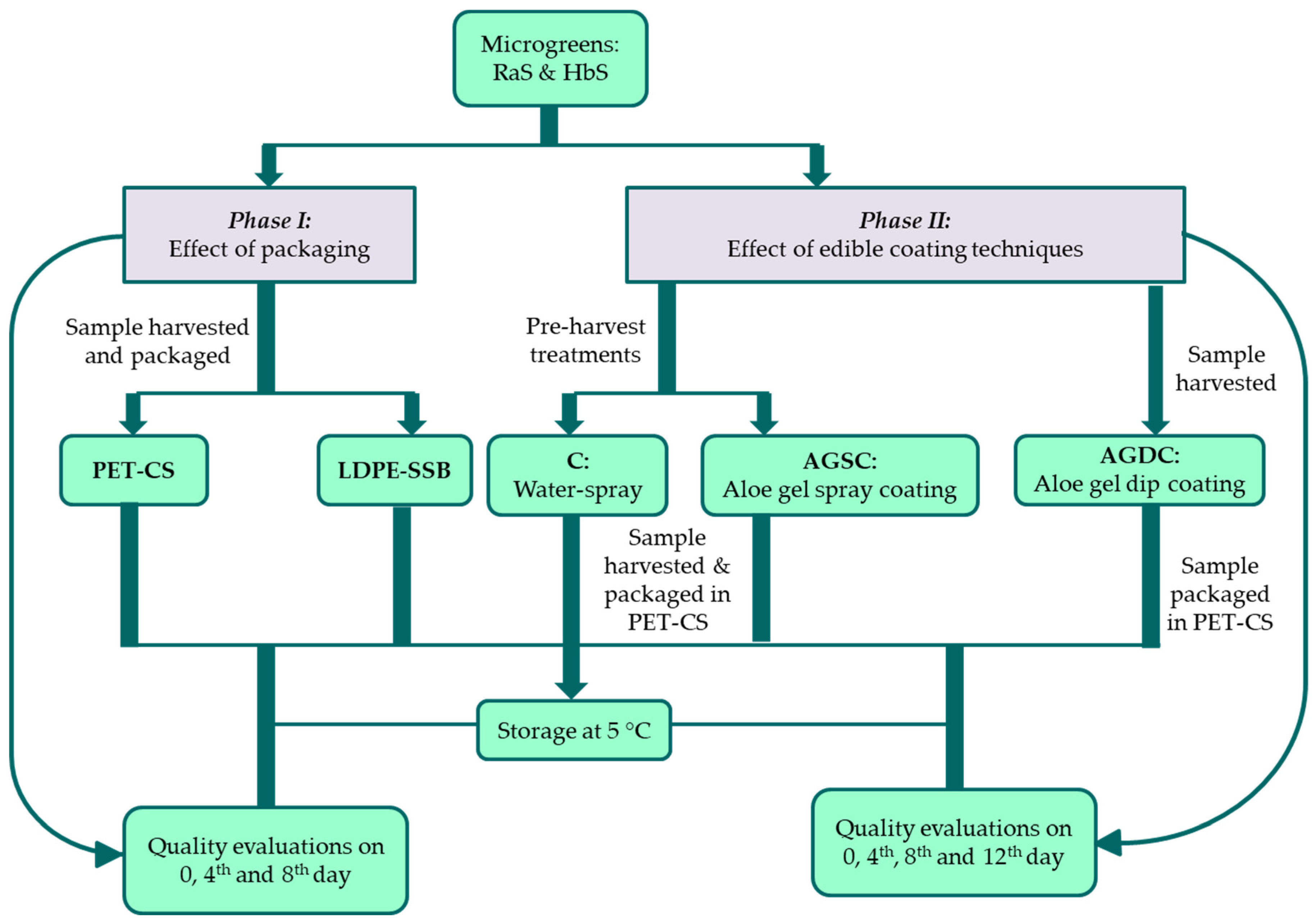
2.2. Experimental Design
2.2.1. Postharvest Packaging—Phase I
2.2.2. Edible Coating Techniques and Application—Phase II
2.3. Quality Evaluations
2.3.1. Physiological Loss in Weight
2.3.2. Respiration Rate
2.3.3. Electrolyte Leakage
2.3.4. Instrumental Colour
2.3.5. Ascorbic Acid
2.3.6. Microbial Enumeration
2.3.7. Overall Acceptability and Marketability
2.3.8. Scanning Electron Microscopy
2.3.9. Statistical Analysis
3. Results
3.1. Effect of Packaging on Postharvest Quality and Shelf Life of Radish (RaS) and Roselle (HbS) Microgreens
3.1.1. Physiological Loss in Weight (PLW)
3.1.2. Respiration Rate (RR)
3.1.3. Electrolyte Leakage (EL)
3.1.4. Instrumental Colour
3.1.5. Microbial Quality
3.1.6. Ascorbic Acid
3.1.7. Overall Acceptability and Marketability
3.2. Comparative Effect of Edible Coating Techniques on Postharvest Quality and Shelf Life of Radish (RaS) and Roselle (HbS) Microgreens
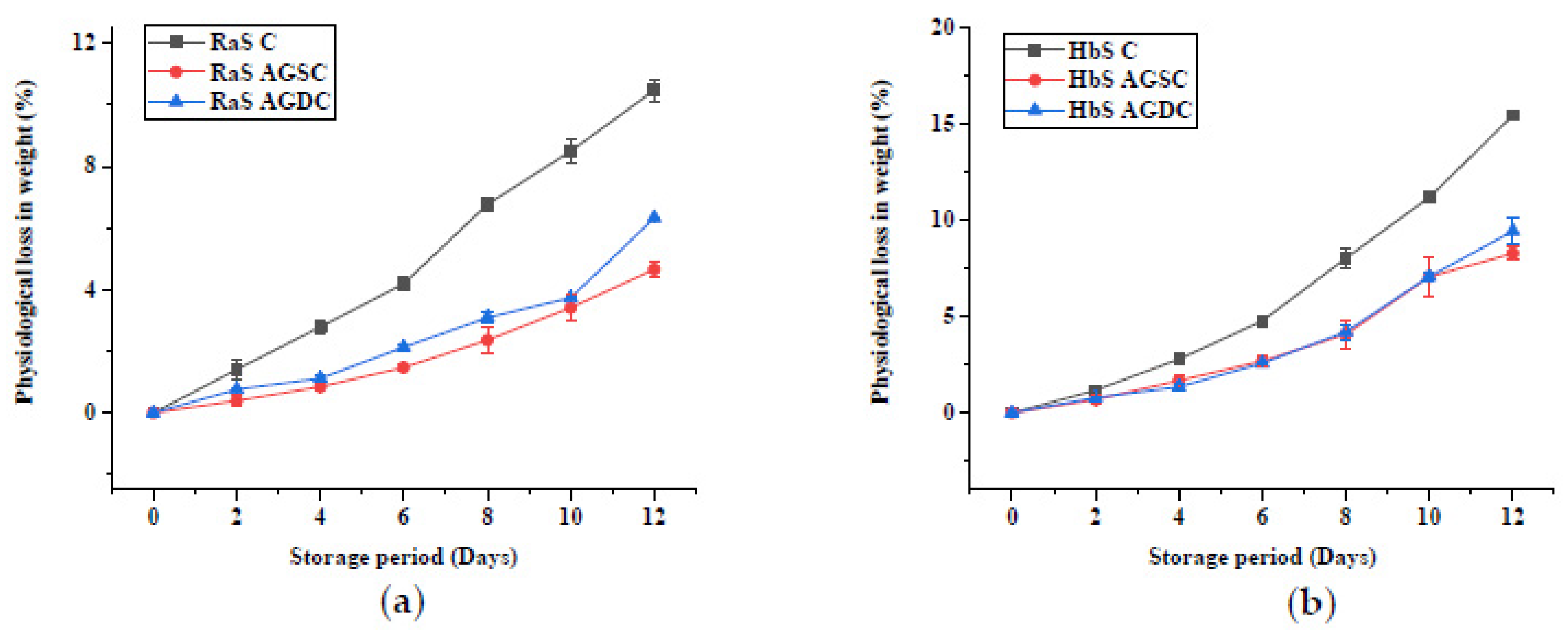
3.2.1. Physiological Loss in Weight
3.2.2. Respiration Rate
3.2.3. Electrolyte Leakage
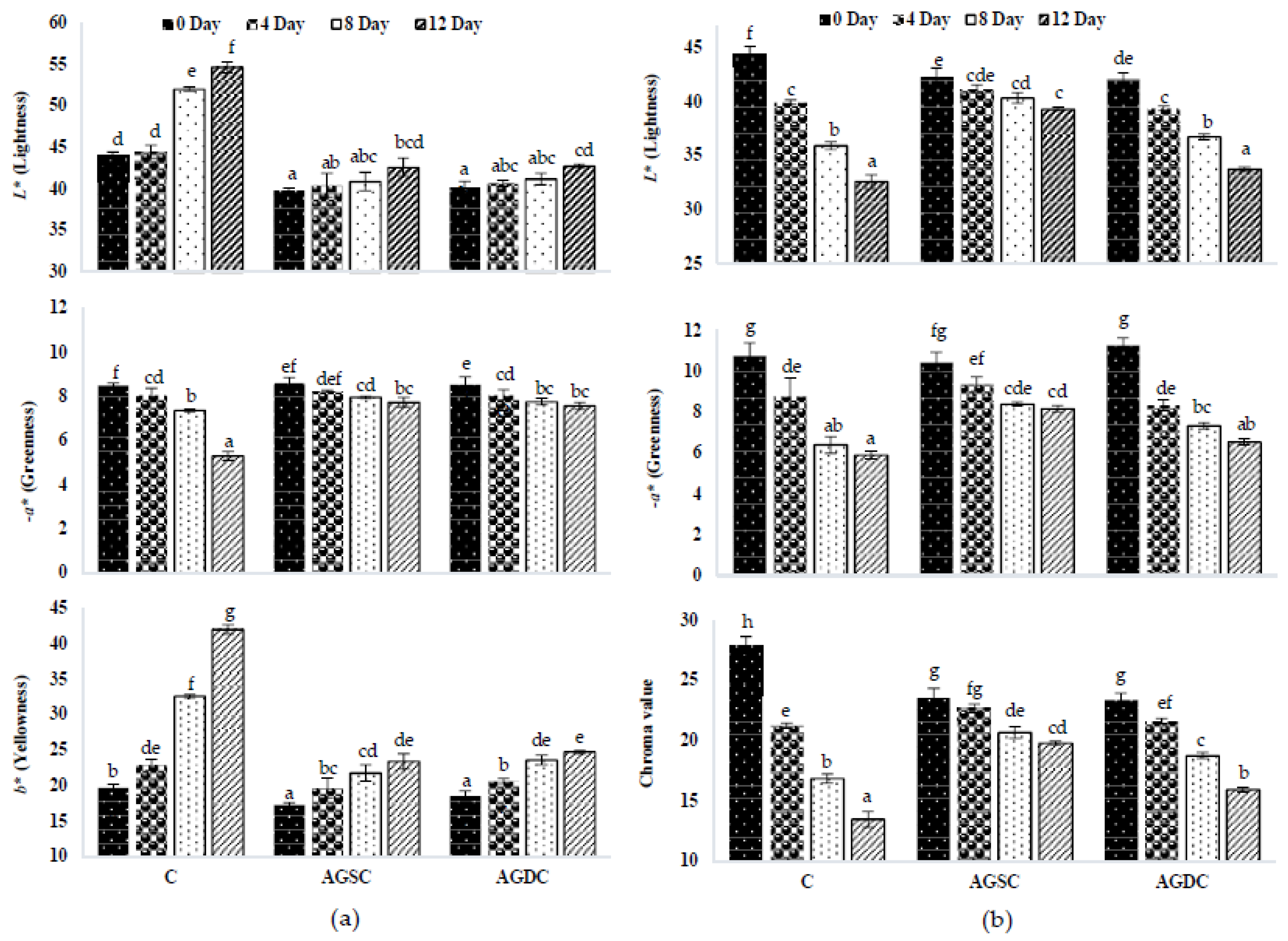
3.2.4. Instrumental Colour
3.2.5. Microbial Quality
3.2.6. Ascorbic Acid
3.2.7. Overall Acceptability and Marketability
3.2.8. Scanning Electron Microscope (SEM) Image Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gumble, J.J.; Berghage, R.; Stearns, D. Production and financial analyses of rotating living wall, an urban agricultural system. J. Environ. Prot. 2015, 6, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Chandra, D.; Kim, J.G.; Kim, Y.P. Changes in microbial population and quality of microgreens treated with different sanitizers and packaging films. Hort. Environ. Biotechnol. 2012, 53, 32–40. [Google Scholar] [CrossRef]
- Kou, L.; Luo, Y.; Yang, T.; Xiao, Z.; Turner, E.R.; Lester, G.E.; Wang, Q.; Camp, M.J. Postharvest biology, quality and shelf life of buckwheat microgreens. LWT-Food Sci. Technol. 2013, 51, 73–78. [Google Scholar] [CrossRef]
- Berba, K.J.; Uchanski, M.E. Post-harvest physiology of microgreens. J. Young Investig. 2012, 24, 1–5. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Xie, Z.K.; Yu, L.L.; Wang, Q. Effect of light exposure on sensorial quality, concentrations of bioactive compounds and antioxidant capacity of radish microgreens during low temperature storage. Food Chem. 2014, 151, 472–479. [Google Scholar] [CrossRef]
- Dalal, N.; Siddiqui, S.; Neeraj. Effect of chemical treatment, storage and packaging on physico-chemical properties of sunflower microgreens. Int. J. Chem. Stud. 2019, 7, 1046–1050. [Google Scholar]
- Techavises, N.; Hikida, Y. Development of a mathematical model for simulating gas and water vapor exchanges in modified atmosphere packaging with macroscopic perforations. J. Food Eng. 2008, 85, 94–104. [Google Scholar] [CrossRef]
- Tudela, J.A.; Marín, A.; Garrido, Y.; Cantwell, M.; Medina-Martínez, M.S.; Gil, M.I. Off-odour development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Avena-Bustillos, R.D.J.; Krochta, J.M.; Saltveit, M.E. Water vapor resistance of red delicious apples and celery sticks coated with edible caseinate-acetylated monoglyceride film. J. Food Sci. 1997, 62, 351–354. [Google Scholar] [CrossRef]
- Tay, S.L.; Perera, C.O. Effect of 1-methylcyclopropene treatment and edible coatings on the quality of minimally processed lettuce. J. Food Sci. 2004, 69, 131–135. [Google Scholar] [CrossRef]
- Fortunati, E.; Giovanale, G.; Luzi, F.; Mazzaglia, A.; Kenny, J.M.; Torre, L.; Balestra, G.M. Effective postharvest preservation of kiwifruit and romaine lettuce with a chitosan hydrochloride coating. Coatings 2017, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Misir, J.; Brishti, F.H.; Hoque, M.M. Aloe vera gel as a novel edible coating for fresh fruits: A review. Am. J. Food Sci. Technol. 2014, 2, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT-Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays post-cut surface browning and maintains quality of cold stored lotus (Nelumbo nucifera Gaertn.) root slices. Sci. Hortic. 2019, 256, 108612. [Google Scholar] [CrossRef]
- Zhao, Y. Application of commercial coatings. In Edible Coatings and Films to Improve Food Quality, 2nd ed.; Baldwin, E.A., Hagenmaier, R., Jinhe, B., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 319–332. [Google Scholar]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Marpudi, S.L.; Abirami, L.S.S.; Srividya, N. Enhancement of storage life and quality maintenance of papaya fruits using Aloe vera based antimicrobial coating. Indian J. Biotechnol. 2011, 10, 83–89. [Google Scholar]
- Marpudi, S.L.; Pushkala, R.; Srividya, N. Aloe vera gel coating for postharvest quality maintenance of fresh fig fruits. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 878–887. [Google Scholar]
- Ali, S.; Khan, A.S.; Nawaz, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Hussain, S. Aloe vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit. Postharvest Biol. Technol. 2019, 157, 110960. [Google Scholar] [CrossRef]
- Pushkala, R.; Parvathy, K.R.; Srividya, N. Chitosan powder coating, a novel simple technique for enhancement of shelf life quality of carrot shreds stored in macro perforated LDPE packs. Innov. Food Sci. Emerg. Technol. 2012, 16, 11–20. [Google Scholar] [CrossRef]
- Pushkala, R.; Raghuram, P.K.; Srividya, N. Chitosan based powder coating technique to enhance phytochemicals and shelf life quality of radish shreds. Postharvest Biol. Technol. 2013, 86, 402–408. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Alcazar, J.; Yang, T.; Luo, Y.; Wang, Q.; Chen, P. Effect of preharvest CaCl2 spray and postharvest UV-B radiation on storage quality of broccoli microgreens, a richer source of glucosinolates. J. Food Compost. Anal. 2018, 67, 55–62. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Liu, X.; Luo, Y. Effects of pre-and postharvest calcium treatments on shelf life and postharvest quality of broccoli microgreens. HortScience 2015, 50, 1801–1808. [Google Scholar] [CrossRef] [Green Version]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, Y.; Lester, G.E.; Kou, L.; Yang, T.; Wang, Q. Postharvest quality and shelf life of radish microgreens as impacted by storage temperature, packaging film, and chlorine wash treatment. LWT-Food Sci. Technol. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Ihl, M.; Aravena, L.; Scheuermann, E.; Uquiche, E.; Bifani, V. Effect of immersion solutions on shelf-life of minimally processed lettuce. LWT-Food Sci. Technol. 2003, 36, 591–599. [Google Scholar] [CrossRef]
- Watada, A.E.; Qi, L. Quality of fresh-cut produce. Postharvest Biol. Technol. 1999, 15, 201–205. [Google Scholar] [CrossRef]
- Mampholo, M.B.; Sivakumar, D.; Van Rensburg, J. Variation in bioactive compounds and quality parameters in different modified atmosphere packaging during postharvest storage of traditional leafy vegetables (Amaranthus cruentus L. and Solanum retroflexum). J. Food Qual. 2015, 38, 1–12. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brandenburg, J.S.; Luo, Y. Modified atmosphere packaging for fresh-cut produce. In Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities; Yahia, E.M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 463–489. [Google Scholar]
- Kader, A.A.; Saltveit, M.E. Respiration and gas exchange. In Postharvest Physiology and Pathology of Vegetables, 2nd ed.; Bartz, J.A., Bretcht, J.K., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2003; Volume 2, pp. 7–29. [Google Scholar]
- Kader, A.A. Modified atmospheres during transport and storage. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Richmond, CA, USA, 2002; pp. 135–144. [Google Scholar]
- Apeland, J. Factors affecting respiration and colour during storage of parsley. Acta Hort. 1971, 20, 43–52. [Google Scholar] [CrossRef]
- Nei, D.; Uchino, T.; Sakai, N.; Tanaka, S.I. Prediction of sugar consumption in shredded cabbage using a respiratory model. Postharvest Biol. Technol. 2006, 41, 56–61. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Rahman, M.A.; Hossain, M.A.; Islam, M.N.; Arfin, M.S. Postharvest quality response of strawberries with aloe vera coating during refrigerated storage. J. Hortic. Sci. Biotech. 2017, 92, 598–605. [Google Scholar] [CrossRef]
- Jiang, Y.; Shiina, T.; Nakamura, N.; Nakahara, A. Electrical conductivity evaluation of postharvest strawberry damage. J. Food Sci. 2001, 66, 1392–1395. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Ashraf, S.; Ali, I.; Butt, S.J. Enhancing storage life of bell pepper by UV-C irradiation and edible coatings. Pak. J. Agric. Sci. 2015, 52, 403–411. [Google Scholar]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The effect of the application of edible coatings on or before ultraviolet treatment on postharvest longan fruits. J. Food Qual. 2017, 5454263, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gnanasekharan, V.; Shewfelt, R.L.; Chinnan, M.S. Detection of color changes in green vegetables. J. Food Sci. 1992, 57, 149–154. [Google Scholar] [CrossRef]
- Supapvanich, S.; Arkajak, R.; Yalai, K. Maintenance of postharvest quality and bioactive compounds of fresh-cut sweet leaf bush (Sauropus androgynus L. M err.) through hot CaCl2 dips. Int. J. Food Sci. Technol. 2012, 47, 2662–2670. [Google Scholar] [CrossRef]
- Wang, C.Y. Effect of heat treatment on postharvest quality of kale, collard and brussels sprouts. In XXV International Horticultural Congress, Part 8; Herregods, M., Ed.; Acta Horticulturae: Brussels, Belgium, 2000; pp. 71–78. [Google Scholar]
- Lunadei, L.; Galleguillos, P.; Diezma, B.; Lleó, L.; Ruiz-Garcia, L. A multispectral vision system to evaluate enzymatic browning in fresh-cut apple slices. Postharvest Biol. Technol. 2011, 60, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zheng, J.; Huang, C.; Zhao, X.; Chen, H.; Sun, Z. Effects of combined aqueous chlorine dioxide and chitosan coatings on microbial growth and quality maintenance of fresh-cut bamboo shoots (Phyllostachys praecox f. prevernalis.) during storage. Food Bioproc. Technol. 2015, 8, 1011–1019. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Ashrita, C.H.; Srividya, N. Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res. (in press). [CrossRef]
- Cantwell, M.; Suslow, T. Fresh-cut fruits and vegetables: Aspects of physiology, preparation and handling that affect quality. In Annual Workshop Fresh-Cut Products: Maintaining Quality and Safety; University of California: Davis, CA, USA, 1999; Volume 5, pp. 1–22. [Google Scholar]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [Green Version]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fizman, S.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Arunkumar, S.; Muthuselvam, M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J. Agric. Sci. 2009, 5, 572–576. [Google Scholar]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K.B. Quality change of apple slices coated with Aloe vera gel during storage. J. Food Sci. 2013, 78, C817–C822. [Google Scholar] [CrossRef] [PubMed]
- Dea, S.; Ghidelli, C.; Pérez-Gago, M.B.; Plotto, A. Coatings for minimally processed fruits and vegetables. In Edible Coatings and Films to Improve Food Quality, 2nd ed.; Baldwin, E.A., Hagenmaier, R., Jinhe, B., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 243–289. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.J.; Fontana, E.; Tibaldi, G.; Nicola, S. Qualitative and physiological response of minimally processed garden cress (Lepidium sativum L.) to harvest handling and storage conditions. J. Food Agric. Environ. 2009, 7, 43–50. [Google Scholar]
- Noichinda, S.; Bodhipadma, K.; Mahamontri, C.; Narongruk, T.; Ketsa, S. Light during storage prevents loss of ascorbic acid, and increases glucose and fructose levels in Chinese kale (Brassica oleracea var. alboglabra). Postharvest Biol. Technol. 2007, 44, 312–315. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. var. Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Saberian, H.; Hamidi-Esfahani, Z.; Abbasi, S. Effect of pasteurization and storage on bioactive components of Aloe vera gel. Nutr. Food Sci. 2013, 43, 175–183. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT-Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. New Zeal. J. Crop. Hort. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe vera gel coating enriched with Fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, J.; Gao, H.; Shen, Y.; Li, C.; Yi, P.; He, X.; Ling, D.; Sheng, J.; Li, J.; et al. Effects of polysaccharide-based edible coatings on quality and antioxidant enzyme system of strawberry during cold storage. Int. J. Polym. Sci. 2017, 9746174, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Produce Business. Rise of the Clamshells. Available online: https://www.producebusiness.com/rise-of-the-clamshells/ (accessed on 20 March 2020).











| Loss of Saleability (%) | Marketability Score |
|---|---|
| 0 < 10 | 5 |
| 10 < 19 | 4 |
| 20 < 29 | 3 |
| 30 < 39 | 2 |
| >40 | 1 |
| Packaging | Storage Period (Days) | Ascorbic Acid (mg/100 g) | |||
|---|---|---|---|---|---|
| FAA | DHA | TAA | DHA/FAA Ratio | ||
| Radish Microgreens | |||||
| PET–CS | 0 | 67.83 ± 0.69 d | 6.15 ± 0.79 a | 73.98 ± 3.82 d | 0.091 a |
| 4 | 55.08 ± 1.03 c | 10.85 ± 1.19 b | 65.93 ± 2.54 c | 0.197 b | |
| 8 | 38.29 ± 0.68 a | 16.30 ± 0.78 cd | 54.59 ± 2.46 a | 0.426 d | |
| LDPE–SSB | 0 | 67.83 ± 0.69 d | 6.15 ± 0.79 a | 73.98 ± 3.82 d | 0.091 a |
| 4 | 49.96 ± 2.26 b | 14.08 ± 2.61 c | 64.04 ± 2.51 bc | 0.284 c | |
| 8 | 38.40 ± 0.76 a | 18.98 ± 0.88 d | 57.38 ± 1.92 ab | 0.495 d | |
| Roselle Microgreens | |||||
| PET–CS | 0 | 98.71 ± 0.44 d | 7.92 ± 0.51 a | 106.62 ± 1.77 d | 0.080 a |
| 4 | 52.58 ± 1.52 c | 15.14 ± 1.75 b | 70.01 ± 3.02 c | 0.289 b | |
| 8 | 28.66 ± 0.84 b | 20.58 ± 2.04 c | 49.24 ± 2.21 a | 0.718 c | |
| LDPE–SSB | 0 | 98.71 ± 0.44 d | 7.92 ± 0.51 a | 106.62 ± 1.77 d | 0.080 a |
| 4 | 48.92 ± 3.18 c | 18.80 ± 3.67 bc | 62.00 ± 0.99 b | 0.390 b | |
| 8 | 24.22 ± 1.78 a | 25.51 ± 1.91 d | 49.73 ± 2.00 a | 1.061 d | |
| Packaging | Storage Period (Days) | Radish Microgreens | Roselle Microgreens | ||
|---|---|---|---|---|---|
| OA | MS | OA | MS | ||
| PET–CS | 0 | 8.3 ± 0.5 c | 5 | 8.4 ± 0.5 c | 5 |
| 4 | 7.8 ± 0.4 b | 4 | 7.6 ± 0.6 b | 4 | |
| 8 | 7.0 ± 0.4 a | 3 | 6.6 ± 0.7 a | 3 | |
| LDPE–SSB | 0 | 8.3 ± 0.5 c | 5 | 8.4 ± 0.5 c | 5 |
| 4 | 7.5 ± 0.5 b | 4 | 7.4 ± 0.5 b | 4 | |
| 8 | 6.7 ± 0.7 a | 3 | 6.2 ± 0.7 a | 2 | |
| Treatments | Storage Period (Days) | Ascorbic Acid (mg/100 g) | |||
|---|---|---|---|---|---|
| FAA | DHA | TAA | DHA/FAA Ratio | ||
| Radish Microgreens | |||||
| RaS C | 0 | 78.56 ± 0.37 g | 6.63 ± 0.43 a | 85.19 ± 1.08 d | 0.084 ab |
| 4 | 56.40 ± 1.13 d | 12.54 ± 1.30 bc | 68.94 ± 1.68 c | 0.223 abc | |
| 8 | 42.22 ± 1.34 b | 17.16 ± 1.54 de | 59.38 ± 2.92 b | 0.408 de | |
| 12 | 26.41 ± 2.41 a | 23.32 ± 2.78 f | 49.74 ± 1.97 a | 0.899 f | |
| RaS AGSC | 0 | 99.88 ± 0.51 j | 8.16 ± 0.59 a | 108.04 ± 4.52 f | 0.082 a |
| 4 | 83.45 ± 1.30 h | 9.68 ± 1.50 ab | 93.13 ± 3.55 e | 0.116 abc | |
| 8 | 70.97 ± 2.02 f | 12.79 ± 1.75 bc | 83.76 ± 2.91 d | 0.181 abc | |
| 12 | 56.25 ± 2.19 d | 14.55 ± 2.53 cd | 70.81 ± 3.36 c | 0.261 cd | |
| RaS AGDC | 0 | 94.07 ± 0.73 i | 8.31 ± 0.84 a | 102.38 ± 1.47 f | 0.088 ab |
| 4 | 72.90 ± 0.90 f | 12.38 ± 1.04 bc | 85.29 ± 3.24 d | 0.170 abc | |
| 8 | 63.49 ± 0.78 e | 15.34 ± 0.90 cd | 78.84 ± 1.88 d | 0.242 bc | |
| 12 | 47.07 ± 0.78 c | 20.12 ± 0.90 ef | 67.18 ± 0.98 c | 0.428 e | |
| Roselle Microgreens | |||||
| HbS C | 0 | 107.67 ± 3.60 h | 7.40 ± 1.26 a | 115.07 ± 2.68 g | 0.069 a |
| 4 | 50.21 ± 1.14 c | 15.00 ± 3.19 c | 65.19 ± 5.09 bc | 0.357 cd | |
| 8 | 31.97 ± 0.51 b | 16.29 ± 0.59 cd | 48.26 ±1.12 a | 0.510 d | |
| 12 | 23.45 ± 2.44 a | 19.66 ± 2.82 de | 43.11 ± 3.06 a | 0.858 e | |
| HbS AGSC | 0 | 127.43 ± 0.84 j | 6.93 ± 0.97 a | 134.36 ± 6.81 h | 0.054 a |
| 4 | 83.63 ± 0.57 g | 9.74 ± 0.57 ab | 93.37 ± 4.01 f | 0.116 b | |
| 8 | 67.41 ± 1.83 e | 14.69 ± 1.58 bcd | 82.10 ± 3.24 def | 0.219 abc | |
| 12 | 52.76 ± 2.02 c | 20.27 ± 1.75 de | 73.03 ± 7.49 bcd | 0.386 cd | |
| HbS AGDC | 0 | 119.99 ± 1.76 i | 6.59 ± 1.53 a | 126.58 ± 2.67 h | 0.055 a |
| 4 | 75.48 ± 1.40 f | 10.57 ± 1.22 abc | 86.05 ± 1.79 df | 0.140 ab | |
| 8 | 58.61 ± 1.19 d | 19.33 ± 1.03 de | 77.93 ± 1.81 cde | 0.330 bcd | |
| 12 | 49.19 ± 1.65 c | 23.84 ± 1.43 e | 62.30 ± 2.60 b | 0.486 d | |
| Edible Coating Technique | Storage Period (Days) | Radish Microgreens | Roselle Microgreens | ||
|---|---|---|---|---|---|
| OA | MS | OA | MS | ||
| C | 0 | 8.3 ± 0.5 f | 5 | 8.4 ± 0.5 f | 5 |
| 4 | 7.8 ± 0.4 de | 4 | 7.6 ± 0.6 cde | 4 | |
| 8 | 7.0 ± 0.4 b | 3 | 6.6 ± 0.7 b | 3 | |
| 12 | 6.4 ± 0.6 a | 2 | 5.8 ± 0.5 a | 1 | |
| AGSC | 0 | 8.4 ± 0.5 f | 5 | 8.5 ± 0.5 f | 5 |
| 4 | 8.1 ± 0.2 ef | 5 | 8.1 ± 0.4 ef | 5 | |
| 8 | 7.9 ± 0.5 cde | 5 | 7.9 ± 0.5 de | 5 | |
| 12 | 7.5 ± 0.6 cd | 4 | 7.4 ± 0.6 cd | 4 | |
| AGDC | 0 | 8.4 ± 0.5 f | 5 | 8.5 ± 0.5 f | 5 |
| 4 | 7.9 ± 0.3 def | 5 | 8.0 ± 0.4 ef | 5 | |
| 8 | 7.5 ± 0.6 cd | 4 | 7.3 ± 0.5 c | 4 | |
| 12 | 7.3 ± 0.5 bc | 4 | 6.7 ± 0.5 b | 3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoora, M.D.; Srividya, N. Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens. Foods 2020, 9, 653. https://doi.org/10.3390/foods9050653
Ghoora MD, Srividya N. Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens. Foods. 2020; 9(5):653. https://doi.org/10.3390/foods9050653
Chicago/Turabian StyleGhoora, Manjula D., and Nagarajan Srividya. 2020. "Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens" Foods 9, no. 5: 653. https://doi.org/10.3390/foods9050653
APA StyleGhoora, M. D., & Srividya, N. (2020). Effect of Packaging and Coating Technique on Postharvest Quality and Shelf Life of Raphanus sativus L. and Hibiscus sabdariffa L. Microgreens. Foods, 9(5), 653. https://doi.org/10.3390/foods9050653





