Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pressurized-Liquid Extraction Procedure (PLE)
2.3. Supercritical CO2 Extraction Procedure (SFE)
2.4. Leaching Process (LEA)
2.5. Identification of Anthocyanins by UHPLC
2.6. Identification of Non-Colored Phenolic Compounds by UPLC-ESI-QToF-MS/MS
2.7. Determination of Total Phenolic Content (TPC) and Total Anthocyanins
2.8. Antidiabetic Potential
2.8.1. α-Amylase and α-Glucosidase Inhibition
2.8.2. Dipeptidyl PeptIdase IV (DPP-IV) Inhibition
2.9. Antioxidant Capacity
2.9.1. DPPH
2.9.2. ABTS
2.10. Anthocyanins and Color Stability Evaluation
2.11. Color Measurements
2.12. Reaction Kinetics of Anthocyanins
2.13. Statistical Analysis
3. Results and Discussion
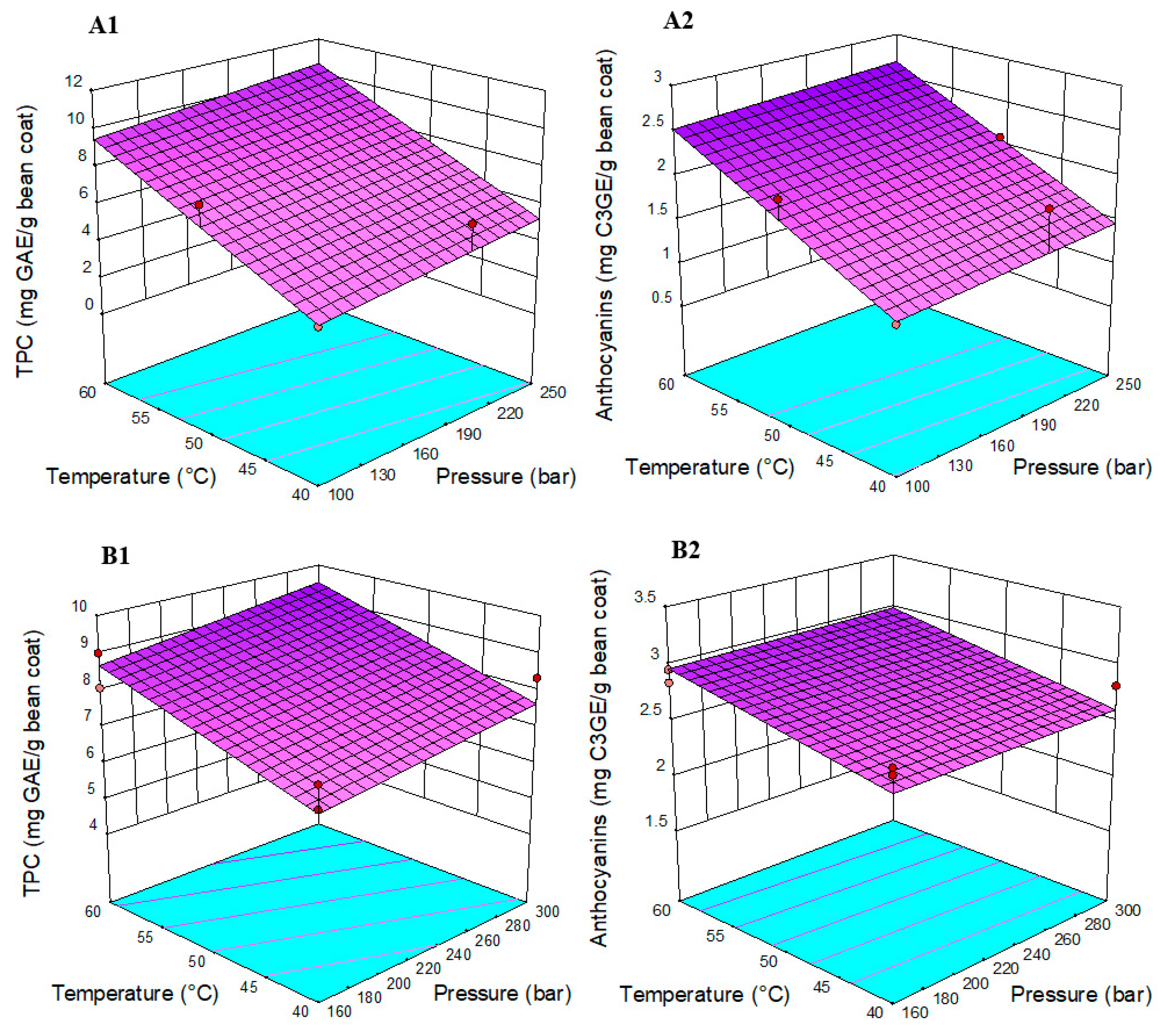
3.1. Effect of Pressurized Liquid Extraction and Supercritical Fluid Extraction on Anthocyanins
3.2. Comparison between Conventional and Alternative Extraction Methods
3.3. Anthocyanins in Common Beans Cultivar San Luis
3.4. Non-Colored Phenolic Compounds in Common Beans Cultivar San Luis
3.5. Biological Potential of the Anthocyanin-Rich Extracts
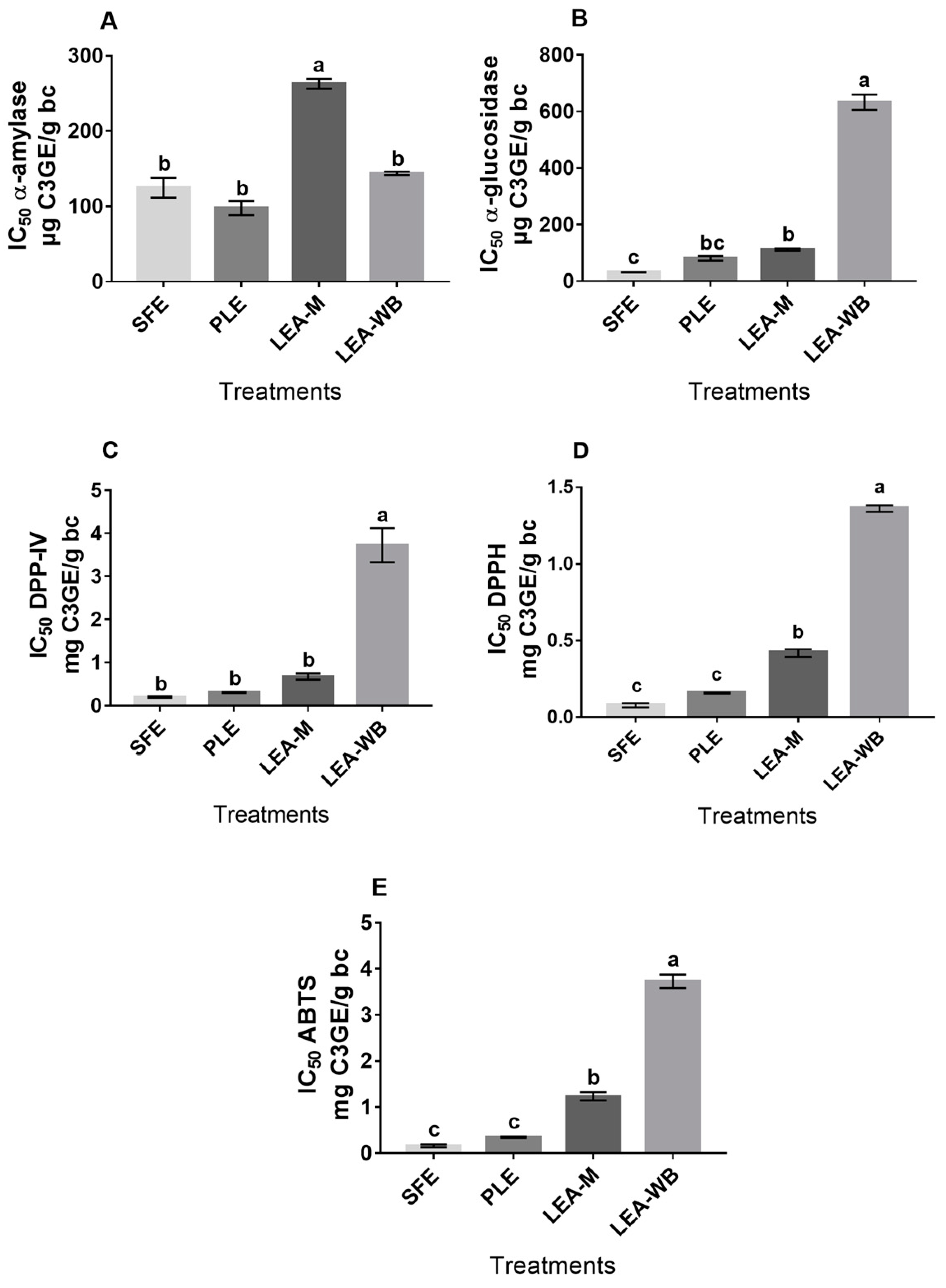
3.5.1. Antidiabetic Potential
3.5.2. Antioxidant Potential
3.6. Stability of the Anthocyanin-Rich Extracts
3.6.1. Effect of Light Exposure
3.6.2. Effect of Storage
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mojica, L.; Meyer, A.; Berhow, M.A.; de Mejía, E.G. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M. Anthocyanins and Diabetes Regulation. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 83–93. ISBN 9780123984562. [Google Scholar]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; De Mejía, E.G.; Martín-Cabrejas, M.A. Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef] [PubMed]
- May, M. Novo awaits green light for diabetes drug. Nat. Biotechnol. 2009, 27, 682–684. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative Extraction Method of Bioactive Compounds from Mulberry (Morus nigra L.) Pulp Using Pressurized-Liquid Extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef]
- Kühn, S.; Temelli, F. Recovery of bioactive compounds from cranberry pomace using ternary mixtures of CO2 + ethanol + water. J. Supercrit. Fluids 2017, 130, 147–155. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Cardenas-Toro, F.P.; Osorio-TobóN, J.F.; Barbero, G.F.; Meireles, M.A.D.A. Obtaining anthocyanin-rich extracts from frozen açai (Euterpe oleracea Mart.) pulp using pressurized liquid extraction. Food Sci. Technol. 2017, 37, 48–54. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Analysis of Flavonoid-Protein Interactions by Advanced Techniques. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 539–543. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Dada, F.A.; Oyeleye, S.I.; Ogunsuyi, O.B.; Olasehinde, T.A.; Adefegha, S.A.; Oboh, G.; Boligon, A.A. Phenolic constituents and modulatory effects of Raffia palm leaf (Raphia hookeri) extract on carbohydrate hydrolyzing enzymes linked to type-2 diabetes. J. Tradit. Complement. Med. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Kim, B.-R.; Kim, H.; Choi, I.; Kim, J.-B.; Jin, C.; Han, A.-R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gonzalez de Mejia, E.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 854–857. [Google Scholar] [CrossRef]
- Rocha, R.P.; Machado, M.; Vaz, M.G.M.V.; Vinson, C.C.; Leite, M.; Richard, R.; Mendes, L.B.B.; Araujo, W.L.; Caldana, C.; Martins, M.A.; et al. Exploring the metabolic and physiological diversity of native microalgal strains (Chlorophyta) isolated from tropical freshwater reservoirs. Algal Res. 2017, 28, 139–150. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and Antioxidant Effects and Phytochemicals of Mulberry Fruit (Morus alba L.) Polyphenol Enhanced Extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef]
- Vukoja, J.; Pichler, A.; Kopjar, M. Stability of Anthocyanins, Phenolics and Color of Tart Cherry Jams. Foods 2019, 8, 255. [Google Scholar] [CrossRef]
- Maciel, L.G.; do Carmo, M.A.V.; Azevedo, L.; Daguer, H.; Molognoni, L.; de Almeida, M.M.; Granato, D.; Rosso, N.D. Hibiscus sabdariffa anthocyanins-rich extract: Chemical stability, in vitro antioxidant and antiproliferative activities. Food Chem. Toxicol. 2018, 113, 187–197. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef] [PubMed]
| Extraction Method | Anthocyanins | Total Phenolic Compounds |
|---|---|---|
| mg C3GE/g Bean Coat | mg GAE/g Bean Coat | |
| PLE | 2.26 ± 0.34 a | 7.05 ± 1.32 bc |
| SFE | 2.64 ± 0.53 a | 11.09 ± 2.43 a |
| LEA-M | 2.43 ± 0.07 a | 8.92 ± 0.3 ab |
| LEA-WB | 1.26 ± 0.05 b | 3.15 ± 0.26 d |
| Peak | Compound | tR (min) | m/z | % Relative Area | |||
|---|---|---|---|---|---|---|---|
| LEA-M | LEA-WB | SFE | PLE | ||||
| 1 | Catechin | 0.770 | 289.1659 | 15.78 | 1.12 | 6.40 | 1.93 |
| 2 | Chlorogenic acid | 0.779 | 353.1818 | 0.29 | 1.56 | 13.52 | 16.28 |
| 3 | Ferulic acid | 0.802 | 193.0495 | 1.66 | 1.84 | 4.41 | 6.90 |
| 4 | Gallic acid * | 3.693 | 169.1064 | 0.10 | 0.14 | 1.97 | 0.15 |
| 5 | Synrigic acid | 3.806 | 197.9050 | 38.65 | 8.44 | 11.85 | 25.04 |
| 6 | Myricetin-3-O-glucoside * | 4.295 | 479.1749 | 2.25 | 0.84 | 2.87 | 5.20 |
| 7 | p-Coumaric acid | 4.343 | 163.1147 | 0.46 | 1.85 | 0.29 | 0.93 |
| 8 | Rutin hydrate | 4.642 | 609.2362 | 1.07 | 39.40 | 9.25 | 9.22 |
| 9 | Myricetin | 5.515 | 317.1280 | 18.06 | 3.35 | 4.92 | 4.54 |
| 10 | Quercetin | 6.229 | 301.1333 | 7.51 | 1.56 | 2.61 | 2.25 |
| 11 | Kaempferol | 7.311 | 285.1339 | 7.08 | 1.51 | 3.15 | 4.05 |
| − | Others | − | − | 7.09 | 38.39 | 38.75 | 23.50 |
| Parameter | Light Condition | Anthocyanins | |
|---|---|---|---|
| LEA-M | SFE | ||
| Rate (k, d−1) | Light | 0.0401 ± 0.001 aB | 0.1297 ± 0.006 aA |
| Dark | 0.0221 ± 9 × 10−4 bB | 0.0824 ± 0.001 bA | |
| Half-life (t1/2, d) | Light | 17.28 ± 0.622 bA | 5.34 ± 0.27 bB |
| Dark | 31.35 ± 1.305 aA | 8.40 ± 0.12 aB | |
| Regression coefficient, R2 | Light | 0.95 | 0.99 |
| Dark | 0.94 | 0.99 | |
| Extraction Method | Light Condition | L* | a* | b* | Color | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | ||
| LEA-M | Light | 91.13 | 91.94 | 18.48 | 13.02 | 0.37 | 3.29 |  |  |
| Dark | 91.13 | 91.36 | 18.48 | 15.09 | 0.37 | 1.64 |  |  | |
| SFE | Light | 90.9 | 95.15 | 18.98 | 7.98 | −0.67 | 2.53 |  |  |
| Dark | 90.9 | 93.48 | 18.98 | 11.66 | −0.67 | 1.73 |  |  | |
| Parameter | Temperature | Anthocyanins | |
|---|---|---|---|
| LEA-M | SFE | ||
| Rate (k, d−1) | 4 °C | 0.0038 ± 5 · 104 bA | 0.0119 ± 9 · 10−4 cA |
| 25 °C | 0.0226 ± 0.0027 abA | 0.0306 ± 5 · 10−4 bA | |
| 32 °C | 0.0388 ± 0.0028 aA | 0.0688 ± 0.0013 aA | |
| Half-life (t1/2, d) | 4 °C | 188.29 ± 25.65 aA | 57.83 ± 4.37 aA |
| 25 °C | 30.62 ± 3.71 bA | 22.63 ± 0.384 bA | |
| 32 °C | 17.81 ± 1.31 bA | 10.07 ± 0.189 cA | |
| Q10 | (4–25 °C) | 2.3546 ± 0.046 aA | 1.8093 ± 0.040 aB |
| (25–32 °C) | 2.1765 ± 0.038 aA | 1.7134 ± 0.034 aB | |
| Energy of activation, Ea (kJ/mol) | 58.7467 ± 1.34 A | 40.6617 ± 1.53 B | |
| Regression coefficient, R2 | 0.99 | 0.99 | |
| Extraction Method | Temperature | L* | a* | b* | Colour | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | ||
| LEA-M | 4°C | 91.13 | 90.52 | 18.48 | 17.57 | 0.37 | −0.07 |  |  |
| 25°C | 91.13 | 91.77 | 18.48 | 12.44 | 0.37 | 3.22 |  |  | |
| 32°C | 91.13 | 93.12 | 18.48 | 8.17 | 0.37 | 6.46 |  |  | |
| SFE | 4°C | 90.9 | 92.15 | 18.98 | 15.14 | −0.67 | 0.13 |  |  |
| 25°C | 90.9 | 94.98 | 18.98 | 7.45 | −0.67 | 2.72 |  |  | |
| 32°C | 90.9 | 96.58 | 18.98 | 4.02 | −0.67 | 3.66 |  |  | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh-Lo, M.; Castillo-Herrera, G.; Mojica, L. Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability. Foods 2020, 9, 655. https://doi.org/10.3390/foods9050655
Hsieh-Lo M, Castillo-Herrera G, Mojica L. Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability. Foods. 2020; 9(5):655. https://doi.org/10.3390/foods9050655
Chicago/Turabian StyleHsieh-Lo, Ming, Gustavo Castillo-Herrera, and Luis Mojica. 2020. "Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability" Foods 9, no. 5: 655. https://doi.org/10.3390/foods9050655
APA StyleHsieh-Lo, M., Castillo-Herrera, G., & Mojica, L. (2020). Black Bean Anthocyanin-Rich Extract from Supercritical and Pressurized Extraction Increased In Vitro Antidiabetic Potential, While Having Similar Storage Stability. Foods, 9(5), 655. https://doi.org/10.3390/foods9050655






