Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient
Abstract
1. Introduction
2. Materials and Methods
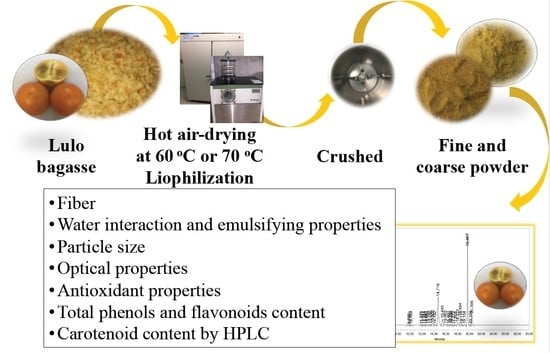
2.1. Lulo Bagasse Preparation
2.2. Dehydration and Milling of Lulo Bagasse
2.3. Analytical Determinations
2.4. Water Interaction and Emulsifying Properties
2.5. Particle Size
2.6. Optical Properties
2.7. Antioxidant Properties
2.8. Total Phenols and Flavonoids Content
2.9. DPPH and ABTS Methods
2.10. Carotenoid Content by HPLC (High-Performance Liquid Chromatography)
2.11. Sorption Isotherms
2.12. Statistical Analysis
3. Results
3.1. Hot Air-Drying of Lulo Bagasse
3.2. Moisture Sorption Isotherms of Lulo Powders
3.3. Physico-Chemical Properties
3.4. Water Interaction and Emulsification Properties of Lulo Bagasse Powders
3.5. Antioxidant Properties
3.6. Carotenoid Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministerio de Agricultura y Desarrollo Rural de Colombia Agronet Producción Nacional por Producto-Lulo. Available online: https://www.agronet.gov.co/Paginas/ProduccionNacionalProducto.aspx (accessed on 24 February 2020).
- Forero, D.P.; Orrego, C.E.; Peterson, D.G.; Osorio, C. Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem. 2015, 169, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.L.; Alter, P.; Dhuique-Mayer, C.; Ruales, J.; Vaillant, F. Identifying carotenoids and phenolic compounds in naranjilla (Solanum quitoense Lam. var. Puyo hybrid), an Andean fruit. J. Agric. Food Chem. 2008, 56, 11890–11899. [Google Scholar] [CrossRef] [PubMed]
- Forero, D.P.; Masatani, C.; Fujimoto, Y.; Coy-Barrera, E.; Peterson, D.G.; Osorio, C. Spermidine derivatives in lulo (Solanum quitoense Lam.) fruit: Sensory (taste) versus biofunctional (ACE-inhibition) properties. J. Agric. Food Chem. 2016, 64, 5375–5383. [Google Scholar] [CrossRef] [PubMed]
- Departamento Nacional de Planeación de Colombia Colombianos Botan 9,76 Millones de Toneladas de Comida al año. Available online: https://www.dnp.gov.co/Paginas/Colombianos-botan-9,76-millones-de-toneladas-de-comida-al-año.aspx (accessed on 21 February 2020).
- De Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts as a potential fat replacer. LWT Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- AOAC. Official method 973.18, 2000. In Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Mertens, D.; Allen, M.; Carmany, J.; Clegg, J.; Davidowicz, A.; Drouches, M.; Frank, K.; Gambin, D.; Garkie, M.; Gildemeister, B.; et al. Gravimetric Determination of Amylase-Treated Neutral Detergent Fiber in Feeds with Refluxing in Beakers or Crucibles: Collaborative Study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Process. Process. Intensif. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agric. Biol. Chem. 1972, 36, 719–727. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.P.; Dexter, D.T.; Aruoma, O.I. Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Res. Int. 2005, 38, 357–367. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J. Agric. Food Chem. 2013, 61, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Camp, J. Van Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.; Spiess, W.E.L.; Jung, G. Standardization of Isotherm Measurements (Cost Project 90 and 90 bis). In Properties of Water in Foods; En Simatos, D., Multon, J.L., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 661–679. [Google Scholar]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Moisture sorption isotherms and isosteric heat of sorption of dry persimmon leaves. Food Biosci. 2014, 7, 88–94. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Vesterlund, S.; Salminen, K.; Salminen, S. Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. Int. J. Food Microbiol. 2012, 157, 319–321. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J.A. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Rodríguez-González, S.; Pérez-Ramírez, I.F.; Loarca-Piña, G.; Amaya-Llano, S.; Gallegos-Corona, M.A.; Reynoso-Camacho, R. Juice by—Products as a source of dietary fibre and antioxidants and their effect on hepatic steatosis. J. Funct. Foods 2015, 17, 93–102. [Google Scholar] [CrossRef]
- Larrauri, J. New approaches in the preparation of high dietary fibre powders from fruit by-products. Trends Food Sci. Technol. 1999, 10, 3–8. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2016, 8, 1462. [Google Scholar] [CrossRef]
- Pérez-Álvarez, J.A.; Viuda-Martos, M.; López-Marcos, M.C.; Fernández-López, J.; Sendra, E.; López-Vargas, J.H. Role of Fiber in Cardiovascular Diseases: A Reviewle. Compr. Rev. Food Sci. Food Saf. 2010, 9, 240–258. [Google Scholar]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Evaluation of Particle Size Influence on Proximate Composition, Physicochemical, Techno-Functional and Physio-Functional Properties of Flours Obtained from Persimmon (Diospyros kaki Trumb.) Coproducts. Plant Foods Hum. Nutr. 2017, 72, 67–73. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, Y.; Eun, J.-B. Physicochemical characteristics of kimchi powder manufactured by hot air drying and freeze drying. Biocatal. Agric. Biotechnol. 2016, 5, 193–198. [Google Scholar] [CrossRef]
- Santos de Sousa, A.; Vilela Borges, S.; Ferreira Magalhães, N.; Vaz Ricardo, H.; Damico Azevedo, A. Spray-Dried Tomato Powder: Reconstitution Properties and Colour. Braz. Arch. Biol. Technol. 2008, 51, 607–614. [Google Scholar] [CrossRef]
- Bakar, J.; Ee, S.C.; Muhammad, K.; Hashim, D.M.; Adzahan, N. Spray-Drying Optimization for Red Pitaya Peel (Hylocereus polyrhizus). Food Bioprocess Technol. 2013, 6, 1332–1342. [Google Scholar] [CrossRef]
- Bhusari, S.N.; Muzaffar, K.; Kumar, P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Ishida, Y.; Shimamoto, T. Molecular characterization of antimicrobial resistance in Salmonella isolated from animals in Japan. J. Appl. Microbiol. 2009, 106, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Torres-León, C.; Ayala-Aponte, A. Evaluación de polvos alimentarios obtenidos de cáscaras de mango (Mangifera indica) como fuente de ingredientes funcionales. Inf. Tecnol. 2015, 26, 41–50. [Google Scholar] [CrossRef]
- Karnik, D.; Wicker, L. Emulsion stability of sugar beet pectin fractions obtained by isopropanol fractionation. Food Hydrocoll. 2018, 74, 249–254. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Y.; Yang, J.; Jiang, N.; Wang, Q.; Chu, D.-T.; Han, Y.; Zhou, J. Thermodynamic sorption properties, water plasticizing effect and particle characteristics of blueberry powders produced from juices, fruits and pomaces. Powder Technol. 2018, 323, 208–218. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Landines, E.F.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying process and particle size of persimmon fibre on its physicochemical, antioxidant, hydration and emulsifying properties. J. Food Sci. Technol. 2017, 54, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- Crozier, S.J.; Preston, A.G.; Hurst, J.W.; Payne, M.J.; Mann, J.; Hainly, L.; Miller, D.L. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem. Cent. J. 2011, 5, 5. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of different drying techniques on physical properties, total polyphenols and antioxidant capacity of blackcurrant pomace powders. LWT Food Sci. Technol. 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, 80–88. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Da Silva, L.M.R.; De Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; De Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Albanese, D.; Adiletta, G.; D’Acunto, M.; Cinquanta, L.; Di Matteo, M. Tomato peel drying and carotenoids stability of the extracts. Int. J. Food Sci. Technol. 2014, 49, 2458–2463. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Abrahamse, L.; Delincée, H.; Adam, S.; Wever, J.; Müller, H.; Rechkemmer, G. Moderate Intervention with Carotenoid-Rich Vegetable Products Reduces Lipid Peroxidation in Men. J. Nutr. 2000, 130, 2200–2206. [Google Scholar] [CrossRef]




| 60 °C | 70 °C | |
|---|---|---|
| First stage: | ||
| k1 | 0.0043 | 0.0048 |
| k2 | 0.0002 | 0.0016 |
| R2 | 0.9176 | 0.9625 |
| Second stage: | ||
| k’1 | 0.0084 | 0.0195 |
| k’2 | −0.0006 | −0.0003 |
| R2 | 0.987 | 0.9319 |
| BET [29] | GAB [26] | ||||||
|---|---|---|---|---|---|---|---|
| W0 | C | R2 | W0 | C | K | R2 | |
| AD60F | 0.050 | 5.902 | 0.992 | 0.113 | −4.103 | −0.614 | 0.929 |
| AD60C | 0.033 | 8.207 | 0.912 | 0.022 | 0.481 | −3.208 | 0.837 |
| AD70F | 0.089 | 0.768 | 0.058 | 0.056 | 0.897 | 1.299 | 0.918 |
| AD70C | 0.060 | 1.208 | 0.061 | 0.063 | 0.937 | 1.108 | 0.929 |
| LYO-F | 0.048 | 2.726 | 0.918 | 0.293 | −4.611 | −0.095 | 0.934 |
| LYO-C | 0.007 | −246.4 | 0.619 | 0.056 | −4.694 | −0.077 | 0.589 |
| AD60F | AD60C | AD70F | AD70C | LYO-F | LYO-C | |
|---|---|---|---|---|---|---|
| aw | 0.119 ± 0.006 a | 0.199 ± 0.006 a | 0.258 ± 0.006 b | 0.267 ± 0.007 b | 0.166 ± 0.003 b | 0.134 ± 0.003 a |
| xw (gw/gsample) | 0.021 ± 0.004 a | 0.035 ± 0.003 a | 0.018 ± 0.002 a,b | 0.015 ± 0.001 b,c | 0.0194 ± 0.0006 c | 0.022 ± 0.002 d |
| xss (gss/gtotal) | 0.236 ± 0.005 e | 0.149 ± 0.012 a | 0.222 ± 0.006 b | 0.146 ± 0.06 c | 0.21 ± 0.11 b | 0.26 ± 0.14 d |
| Fiber content | ||||||
| Hemicellulose (%) | 5.2 ± 0.2 a | 11.3 ± 0.2 e | 5.5 ± 0.2 a | 11.30 ± 0.4 c | 10.3 ± 0.2 b | 10.7 ± 0.4 b,c |
| Cellulose (%) | 18.6 ± 0.3 a | 24.7 ± 0.4 c | 21.3 ± 0.7 b | 24.59 ± 0.05 c | 22.1 ± 0.2 b | 24.6 ± 0.3 c |
| Lignin (%) | 10.1 ± 0.3 a | 20.84 ± 3 b | 17.6 ± 0.23 a | 10.6 ± 0.2 a | 8.5 ± 0.5 b | 10.8 ± 0.4 a |
| Insoluble fiber (%) | 35.2 ± 0.1 b | 40.3 ± 0.4 d | 33.3 ± 0.2 a | 34.2 ± 0.2 a,b | 38.4 ± 0.3 c | 41.65 ± 1.01 e |
| Total fiber (% | 42.6 ± 0.04 b | 50.6 ± 0.6 f | 41.4 ± 0.4 a | 46.5 ± 0.3 c | 46.0 ± 0.4 d | 48.1 ± 0.3 e |
| Colour | ||||||
| L* | 58.4 ± 0.2 c | 50.8 ± 0.2 b | 53.3 ± 0.3 c | 50.97 ± 0.06 b | 63.134 ± 0.13 d | 60.59 ± 0.05 d |
| a* | 10.37 ± 0.02 c | 10.22 ± 0.07 c | 10.35 ± 0.11 c | 10.76 ± 0.13 d | 9.46 ± 0.07 a | 9.98 ± 0.05 b |
| b* | 38.22 ± 0.11 d | 40.5 ± 0.3 f | 39.5 ± 0.2 e | 38.043 ± 0.10 d | 36.74 ± 0.11 c | 34.57 ± 0.06 b |
| C | 39.60 ± 0.01 d | 42.6 ± 0.3 f | 40.8 ± 0.2 e | 39.53 ± 0.12 d | 37.94 ± 0.09 c | 35.98 ± 0.06 a |
| h | 74.84 ± 0.07 c,d | 71.92 ± 0.04 b | 75.32 ± 0.09 d | 74.2 ± 0.2 c | 75.56 ± 0.14 d | 73.70 ± 0.02 c |
| AD60F | AD60C | AD70F | AD70C | LYO-F | LYO-C | |
|---|---|---|---|---|---|---|
| Solubility (%) | 35 ± 5 b | 27 ± 6 b | 32 ± 2 d | 19 ± 4 c | 45 ± 8 a | 30 ± 4 c |
| Higroscopicity (gwater/100 g) | 30.9 ± 0.4 c | 23.0 ± 0.2 a | 23.0 ± 0.2 a | 22.7 ± 1.1 a | 22.1 ± 0.20 a | 25.32 ± 1.1 b |
| Wettability (s) | 31.7 ± 0.6 b | 8.7 ± 1.2 a | 10.0 ± 1.0 a | 11.0 ± 1.7 a | 19.5 ± 2.6 c | 17.0 ± 1.0 b |
| Swelling capacity (mLwater/g) | 4.98 ± 0.02 b | 4.46 ± 0.04 a | 4.97 ± 0.02 b | 4.98 ± 0.05 b | 7.46 ± 0.05 d | 5.48 ± 0.02 c |
| Water holding capacity (gwater/gdry matter) | 5.89 ± 0.10 a | 5.7 ± 0.1 a | 6.3 ± 0.2 a | 7.6 ± 0.8 b | 8.2 ± 0.7 b | 6.4 ± 0.5 a |
| Water retention capacity (gwater/gdry matter) | 4.75 ± 0.02 a | 4.5 ± 0.2 a | 5.5 ± 0.2 b | 5.83 ± 0.06 c,d | 5.9 ± 0.1 d | 5.9 ± 0.4 b,c |
| Emulsifying properties | ||||||
| Oil retention capacity (goil/gsample) | 0.142 ± 0.004 a | 0.18 ± 0.03 a,b | 0.20 ± 0.02 b,c | 0.20 ± 0.02 b | 0.24 ± 0.01 c | 0.45 ± 0.04 d |
| Emulsification activity | N.D | N.D | N.D | N.D | N.D | N.D |
| Emulsification stability | N.D | N.D | N.D | N.D | N.D | N.D |
| AD60F | AD60C | AD70F | AD70C | LYO-F | LYO-C | |
|---|---|---|---|---|---|---|
| β-cryptoxanthin | 4.761 ± 0.014 c | 4.06 ± 0.12 b | 1.197 ± 0.008 a | 1.193 ± 0.011 a | 8.83 ± 0.04 d | 8.72 ± 0.18 d |
| α-carotene | 1.60 ± 0.07 b | 1.577 ± 0.013 b | 0.546 ± 0.017 a | 0.581 ± 0.015 a | 1.75 ± 0.01 c | 1.73 ± 0.03 c |
| β-carotene | 45.2 ± 0.4 b | 45.1 ± 0.3 b | 27.8 ± 0.3 a | 27.61 ± 0.02 a | 61.85 ± 0.25 d | 61.15 ± 0.09 c |
| Total | 51.5 ± 0.5 b | 50.7 ± 0.2 b | 29.51± 0.2 a | 29.38 ± 0.2 a | 72. 6 ± 0.4 d | 71.6 ± 0.3 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinestroza-Córdoba, L.I.; Duarte Serna, S.; Seguí, L.; Barrera, C.; Betoret, N. Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient. Foods 2020, 9, 723. https://doi.org/10.3390/foods9060723
Hinestroza-Córdoba LI, Duarte Serna S, Seguí L, Barrera C, Betoret N. Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient. Foods. 2020; 9(6):723. https://doi.org/10.3390/foods9060723
Chicago/Turabian StyleHinestroza-Córdoba, Leidy Indira, Stevens Duarte Serna, Lucía Seguí, Cristina Barrera, and Noelia Betoret. 2020. "Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient" Foods 9, no. 6: 723. https://doi.org/10.3390/foods9060723
APA StyleHinestroza-Córdoba, L. I., Duarte Serna, S., Seguí, L., Barrera, C., & Betoret, N. (2020). Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient. Foods, 9(6), 723. https://doi.org/10.3390/foods9060723